Ilaprazole enteric coated tablet and preparation method thereof
A technology of ilaprazole and ilaprazole calcium, which is applied in the direction of pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of poor reproducibility of enteric-coated effects, changes in drug release behavior of preparations, Affect the clinical efficacy and other issues, to achieve the effect of improving drug bioavailability, eliminating irritation, and releasing rapidly and stably
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
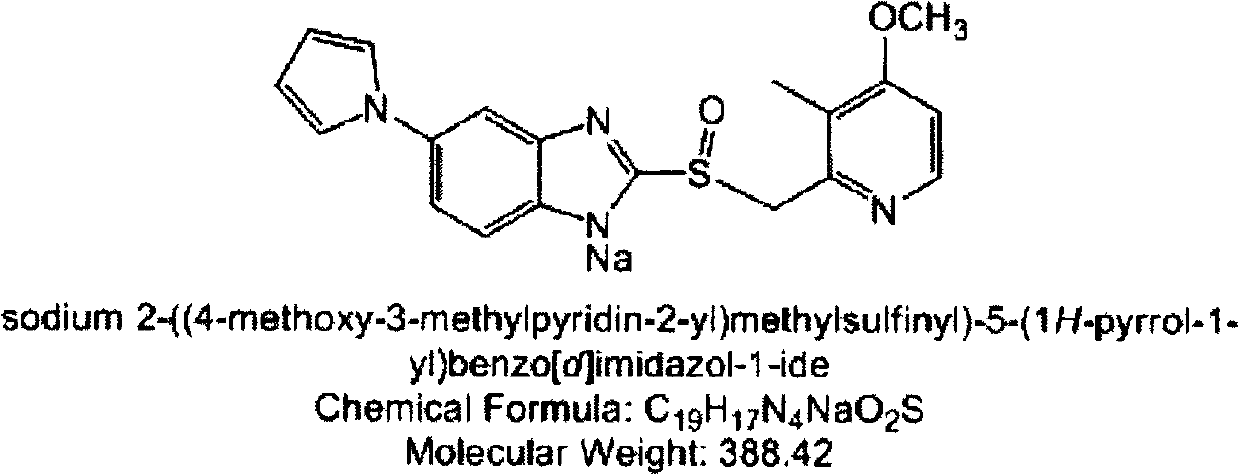
[0036] Embodiment 1: Extrusion spheronization method prepares the pellet core containing ilaprazole sodium
[0037] Table 1 contains the pellet core prescription of ilaprazole sodium
[0038] material
amount (g)
Microcrystalline Cellulose pH101
320
80
72
62
Hydroxypropyl Methyl Cellulose 5cp
10
water
120
[0039] Weigh Mcc pH101 and pregelatinized starch, mix through a 60-mesh sieve; weigh the active ingredient (i.e. ilaprazole sodium) and sodium hydroxide, mix through a 60-mesh sieve; mix the above two. Weigh 5cp of hydroxypropyl methylcellulose (HPMC) and disperse it with a small amount of hot water, then add the remaining amount of water to dissolve it, and make pulp (8% (m / m)). The above-mentioned mixed material and slurry are made into a soft material, and the soft material is extruded and spheronized. The operating ...
Embodiment 2
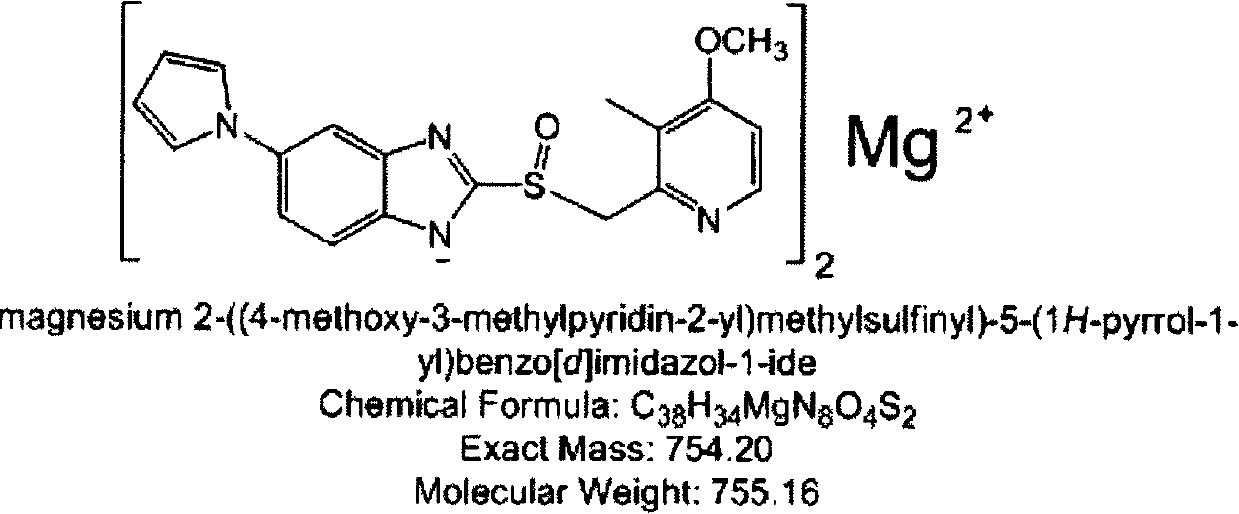
[0042] Embodiment 2: Extrusion spheronization method prepares the pellet core containing ilaprazole magnesium
[0043] Table 3 contains the prescription of micropellet core of ilaprazole magnesium
[0044] material
[0045] Weigh Mcc pH101 and starch, mix through 80-mesh sieve; weigh active ingredient (i.e. ilaprazole magnesium) and magnesium hydroxide, mix through 80-mesh sieve; mix the above two. Weigh 15 cp of hydroxypropyl methylcellulose (HPMC) and disperse it with a small amount of hot water, then add the remaining amount of water to dissolve it, and make pulp (5% (m / m)). The above-mentioned mixed material and slurry are made into a soft material, and the soft material is extruded and spheronized. The main parameters are controlled as follows: the particle size die is 0.2mm, the extrusion speed is 20-30rpm, and the spheronization speed is 2000-2500rpm. 522.4 g of light yellow pellet cores were obtained, with a yield of 99.7%. Direct drying or fluidized bed d...
Embodiment 3
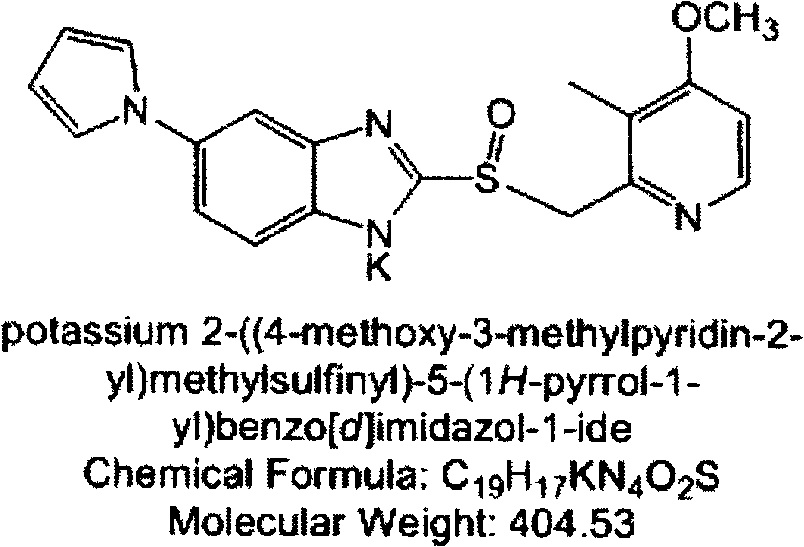
[0046] Embodiment 3: Extrusion spheronization method prepares the pellet core containing ilaprazole lithium
[0047] Table 4 contains the pellet core prescription of ilaprazole lithium
[0048] material
[0049] Weigh Mcc pH101 and lactose, mix through a 100-mesh sieve; weigh the active ingredient (i.e. ilaprazole lithium) and sodium carbonate, mix through a 100-mesh sieve; mix the above two. Povidone K30 was weighed, dissolved in 100 g of 25% (v / v) ethanol aqueous solution, and made into slurry (5% (m / m)). The above-mentioned mixed material and slurry are made into a soft material, and the soft material is extruded and spheronized. The main parameters are controlled as follows: the particle size die is 0.4mm, the extrusion speed is 20-40rpm, and the spheronization speed is 1500-2500rpm. 535.8 g of pellet cores were obtained with a yield of 99.8%. Direct drying or fluidized bed drying at 30°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com