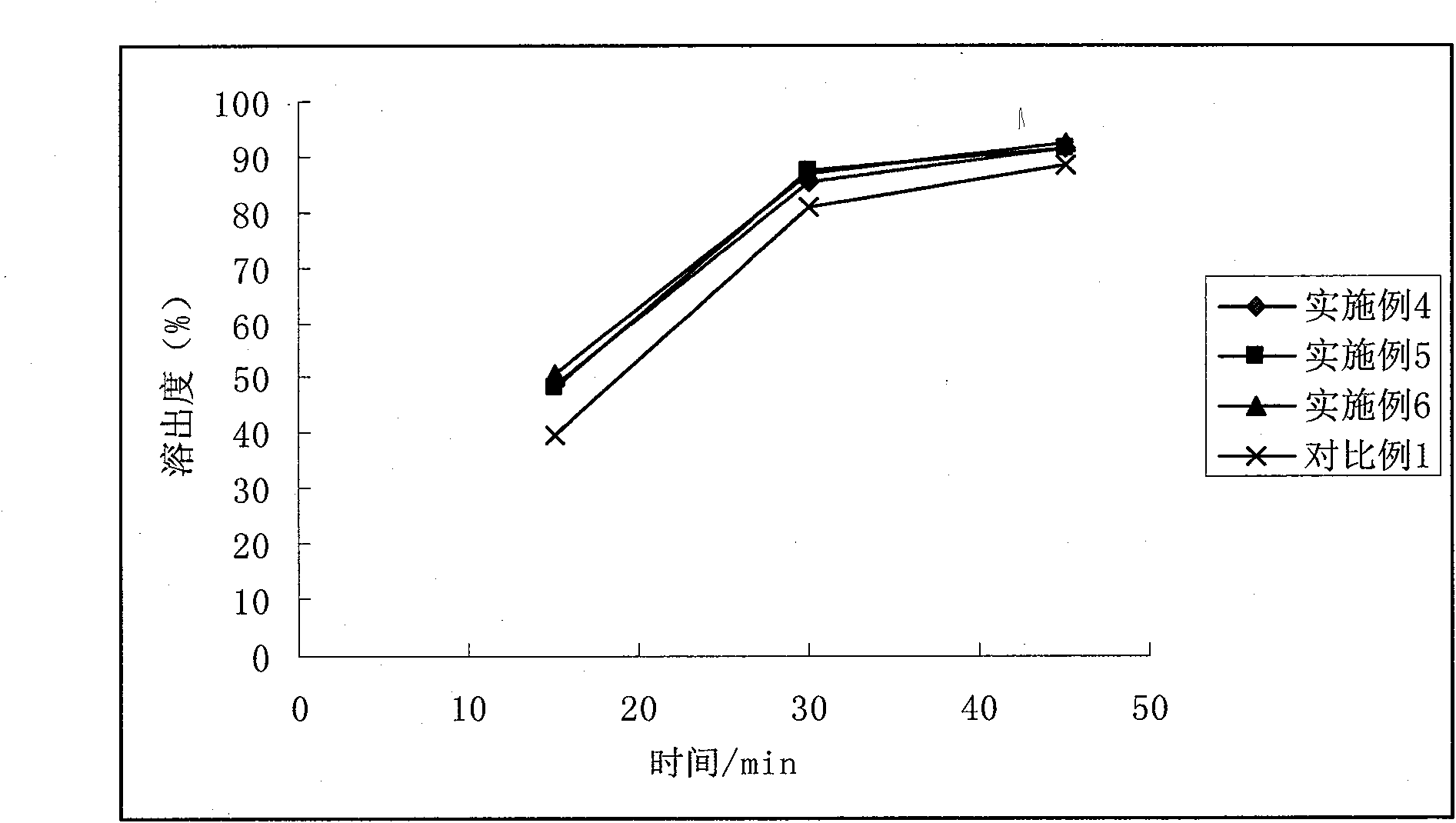
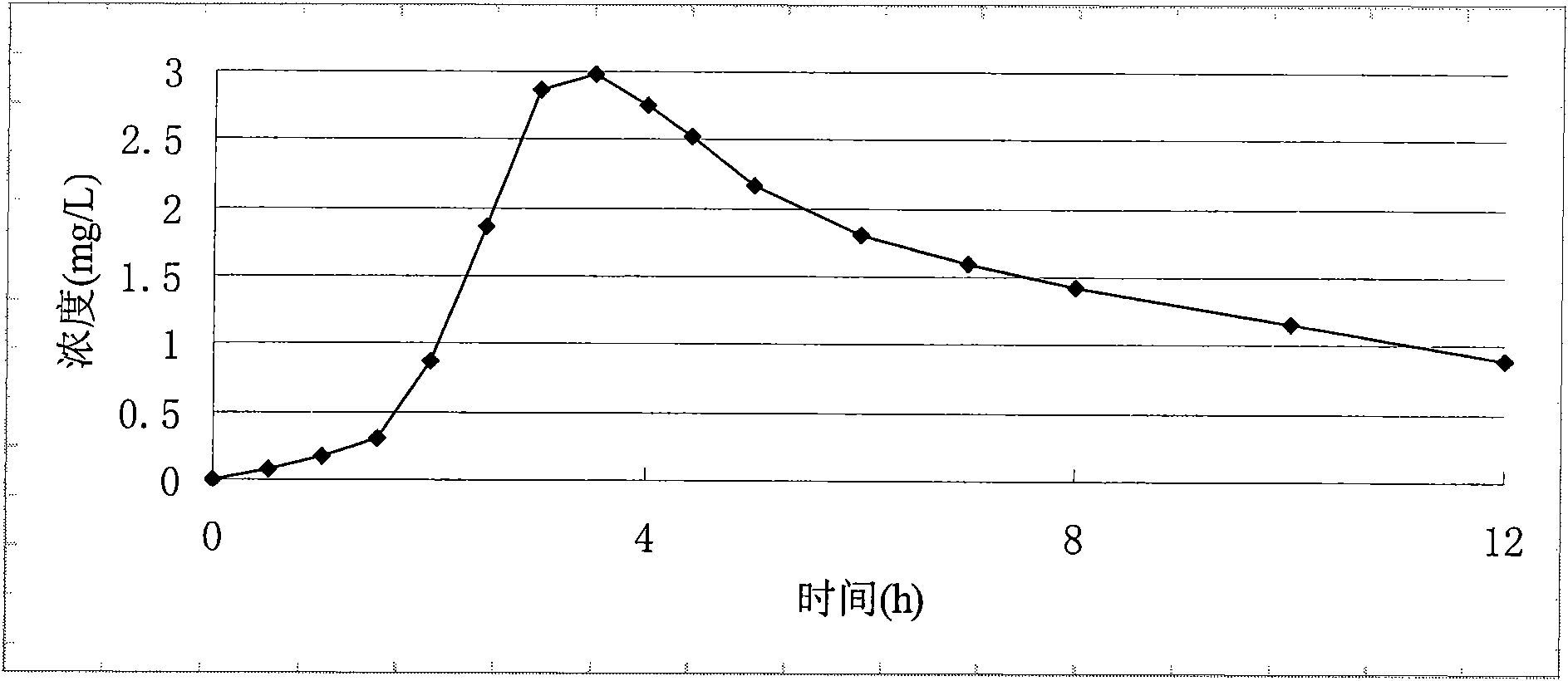
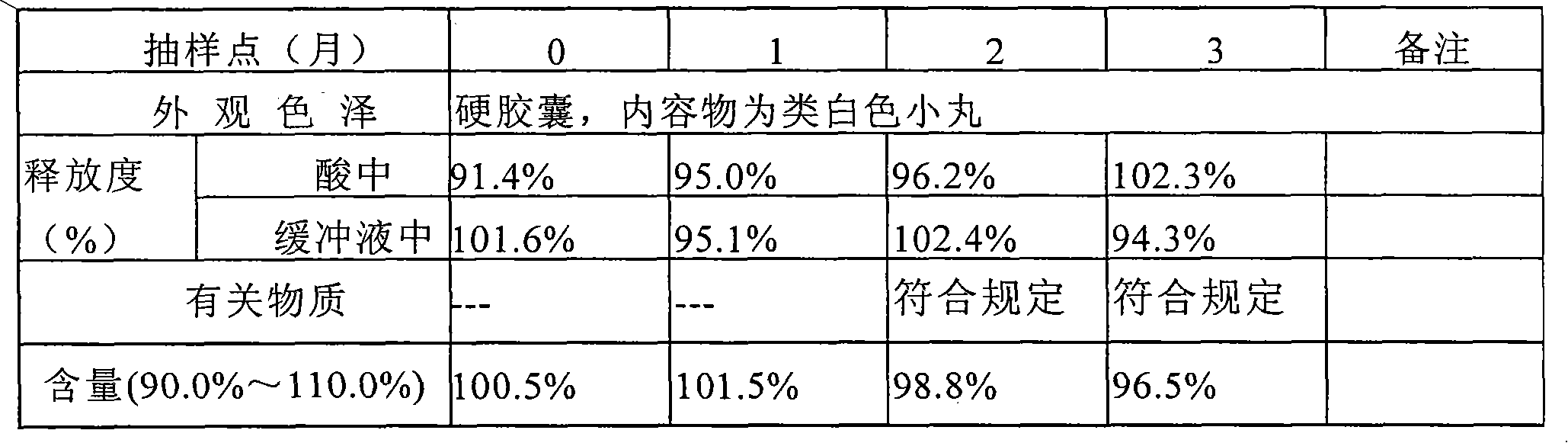
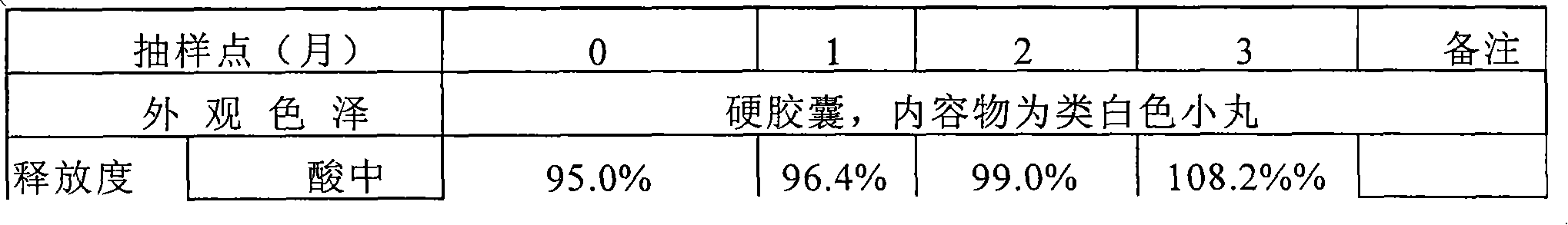
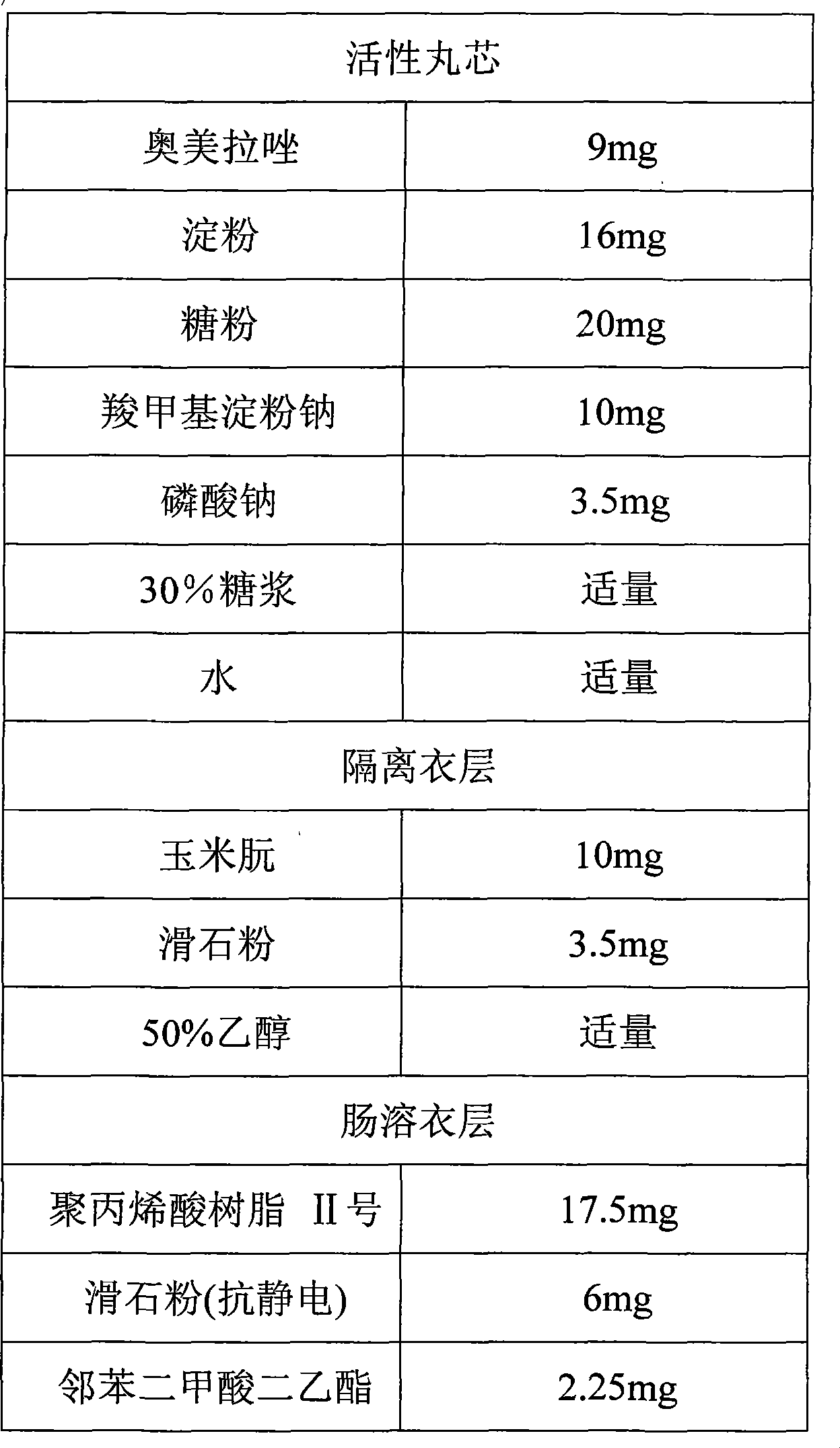
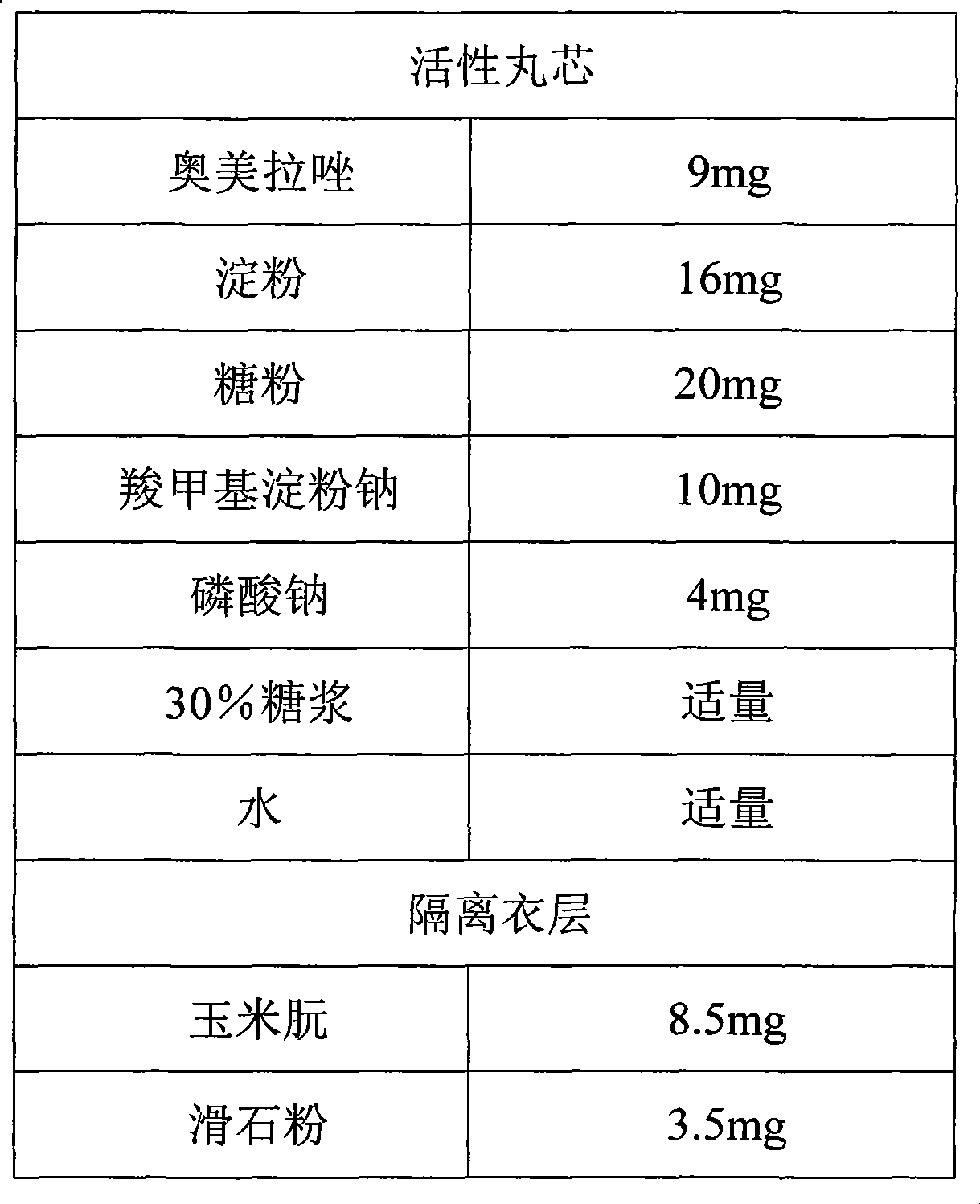
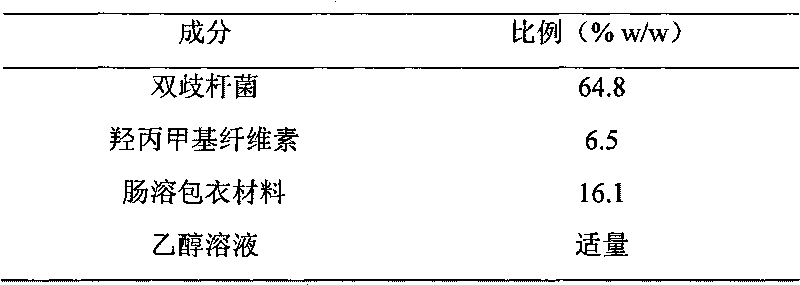
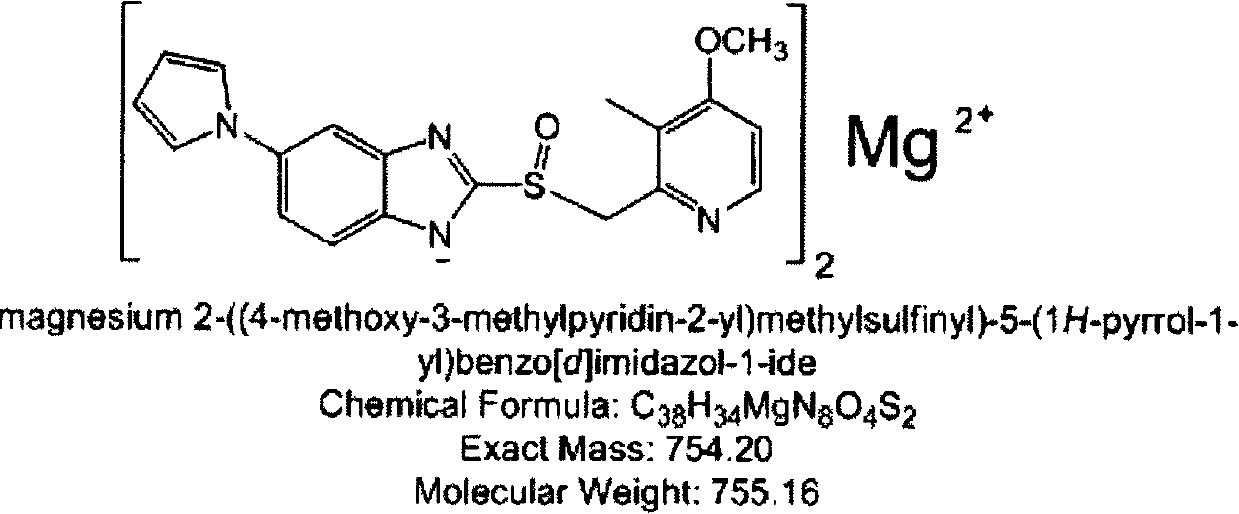
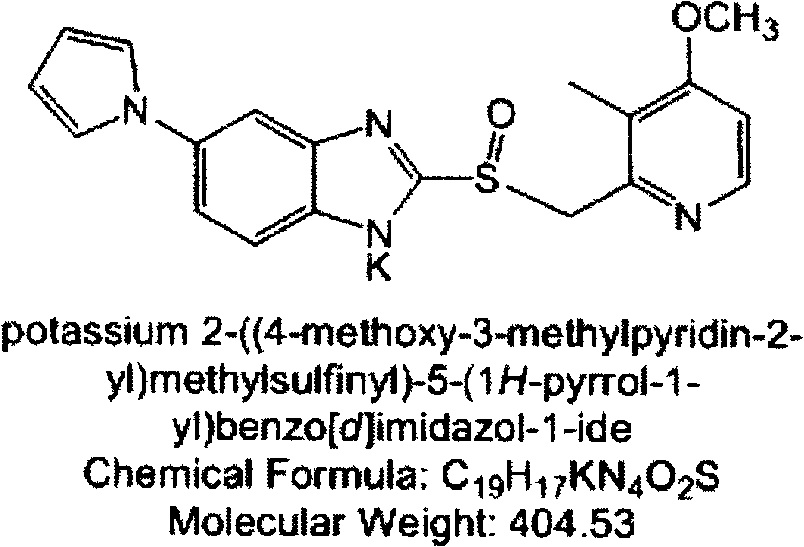
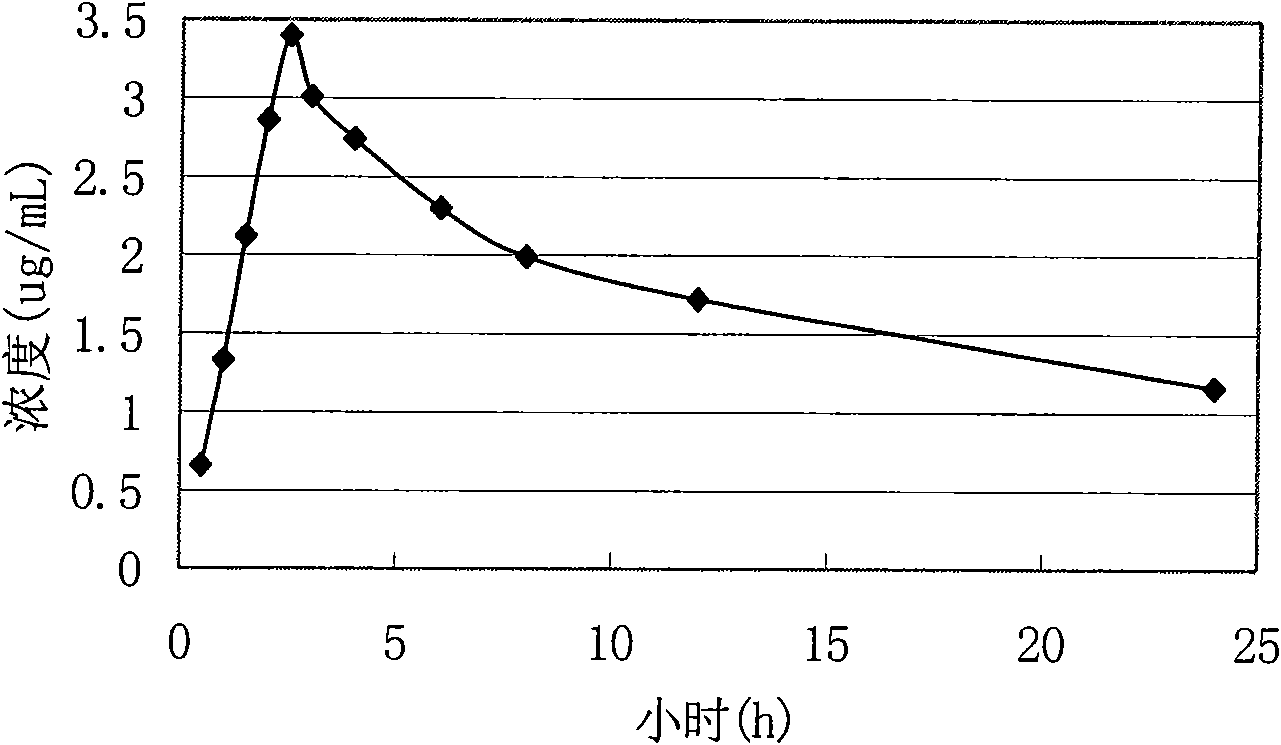
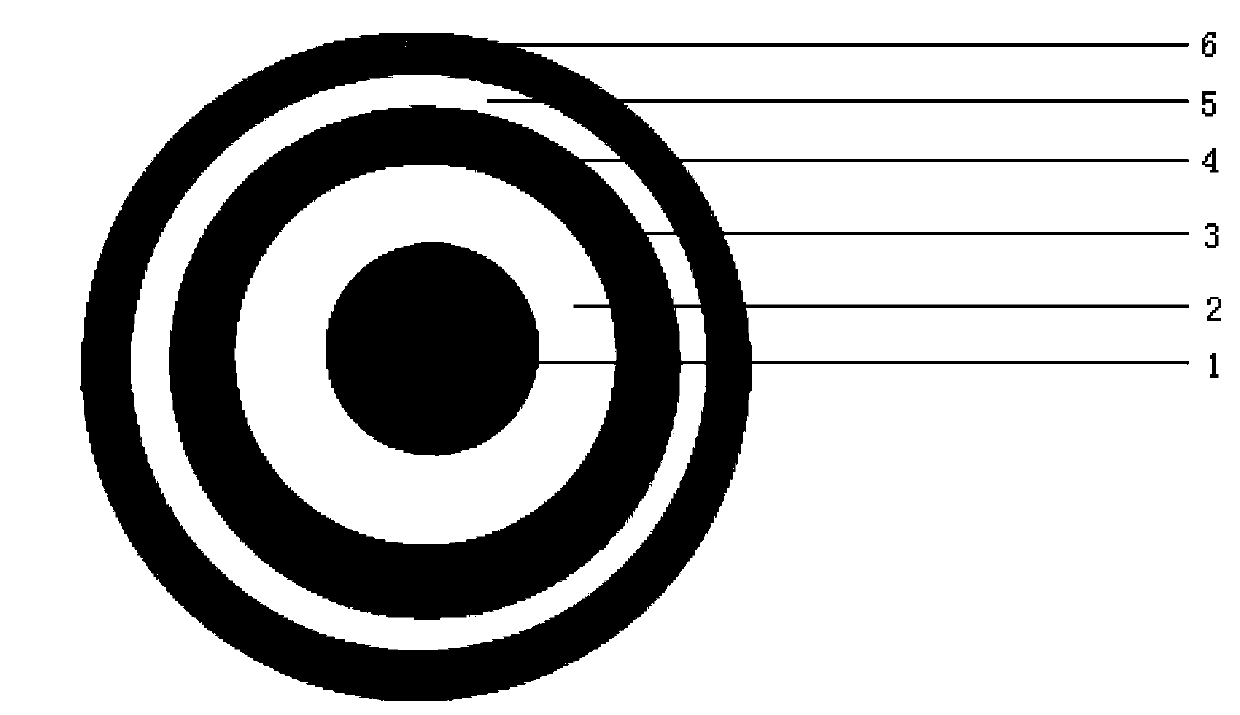
Patents
Literature
116 results about "Enteric coated pellets" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Solid Dosage Form Comprising Proton Pump Inhibitor and Suspension Made Thereof
InactiveUS20080020053A1Stable levelConvenient coatingAntibacterial agentsPowder deliveryBULK ACTIVE INGREDIENTSolid Dose Form
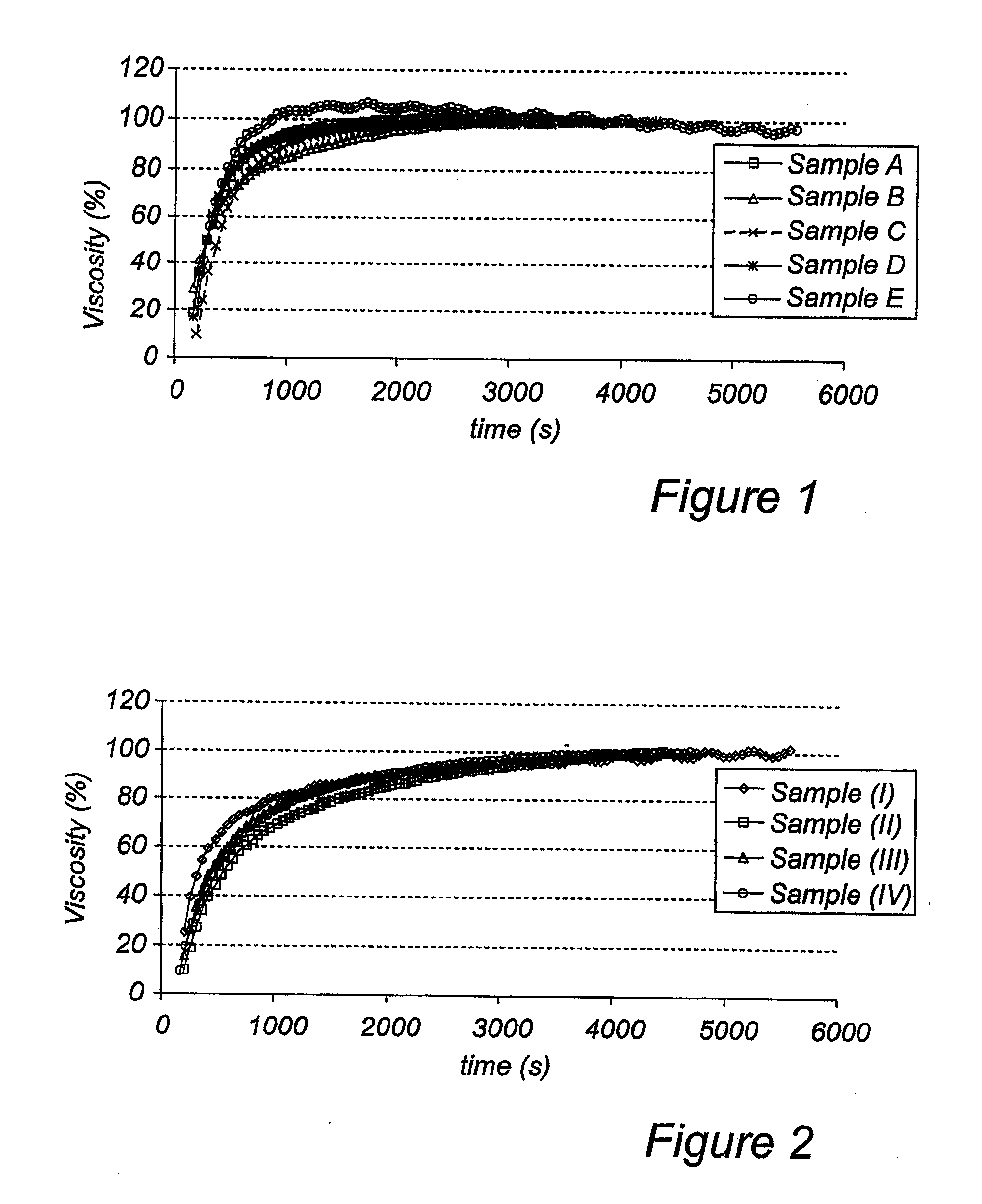
A solid rapidly gelling oral pharmaceutical dosage form, as well as aqueous suspensions prepared thereof, comprising an acid sensitive proton pump inhibitor as active ingredient distributed in a multitude of enteric coated pellets and a suspension modifying granulate comprising a rapidly dissolving diluent granulated together with a gelling agent chosen among xanthan gums, and an acidic pH-regulating agent and a binder. The suspension modifying granulate is rapidly disintegrating and gelling when suspended in an aqueous medium and thus forming a homogenous stable and robust suspension having a reproducible and stable viscosity. Furthermore the invention relates to an improved process for its manufacture and the use of such formulation in medical treatment including prevention of gastrointestinal disorders in humans.
Owner:ASTRAZENECA AB
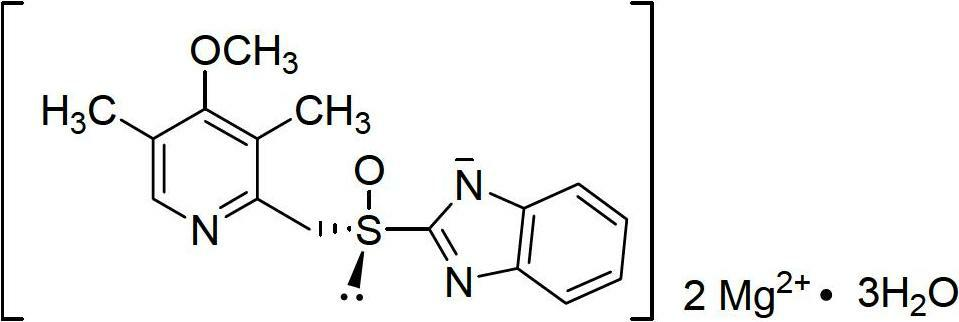
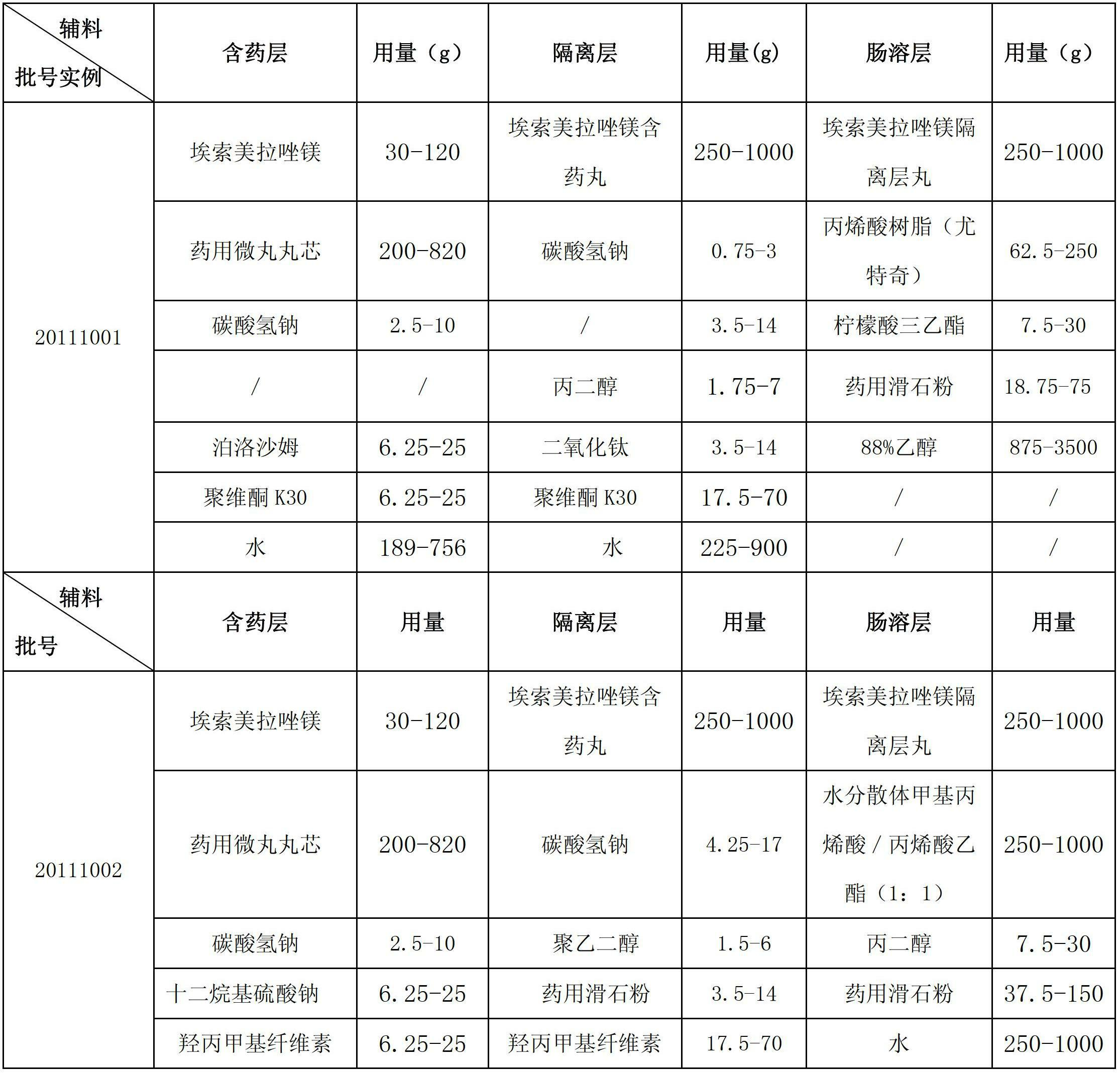
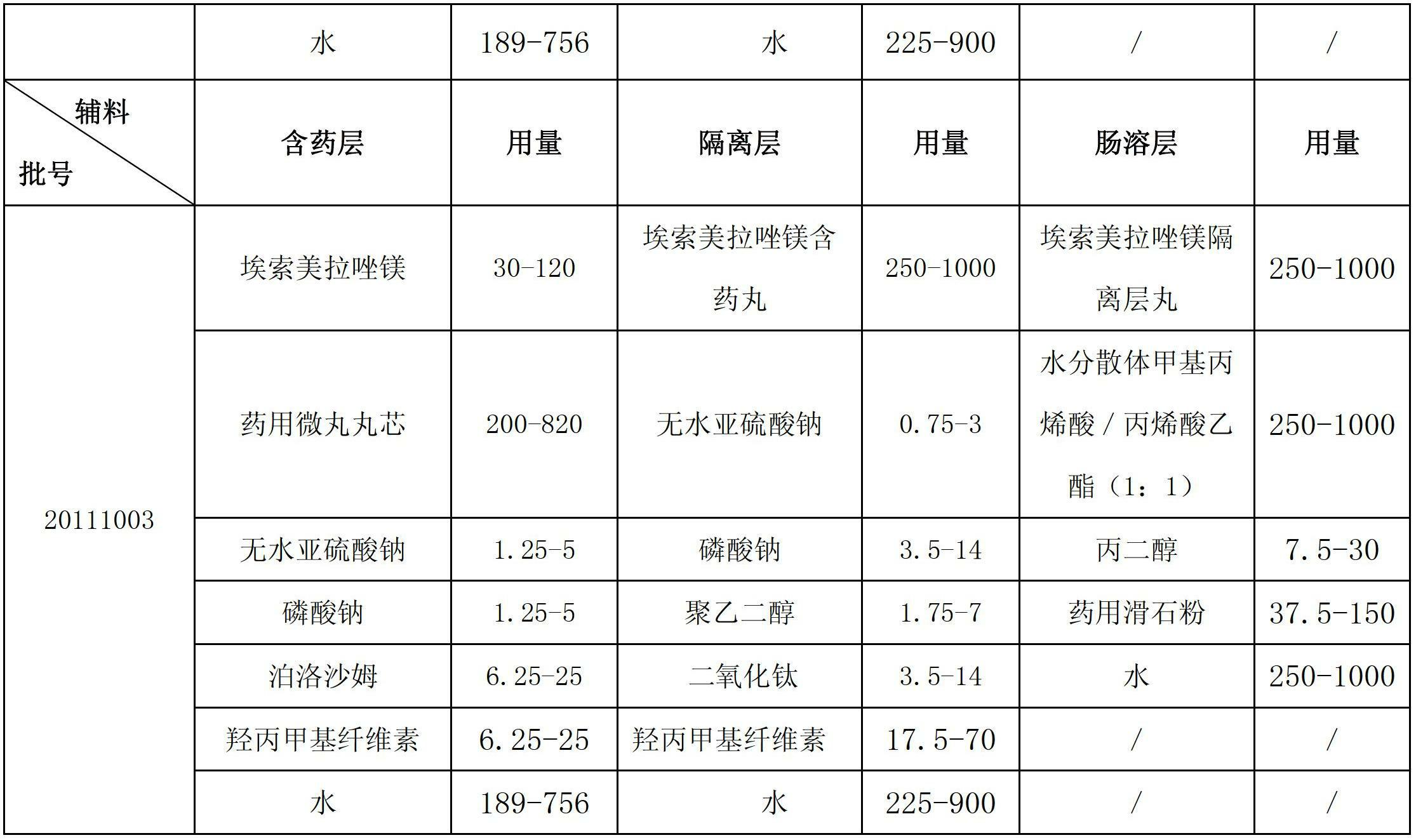
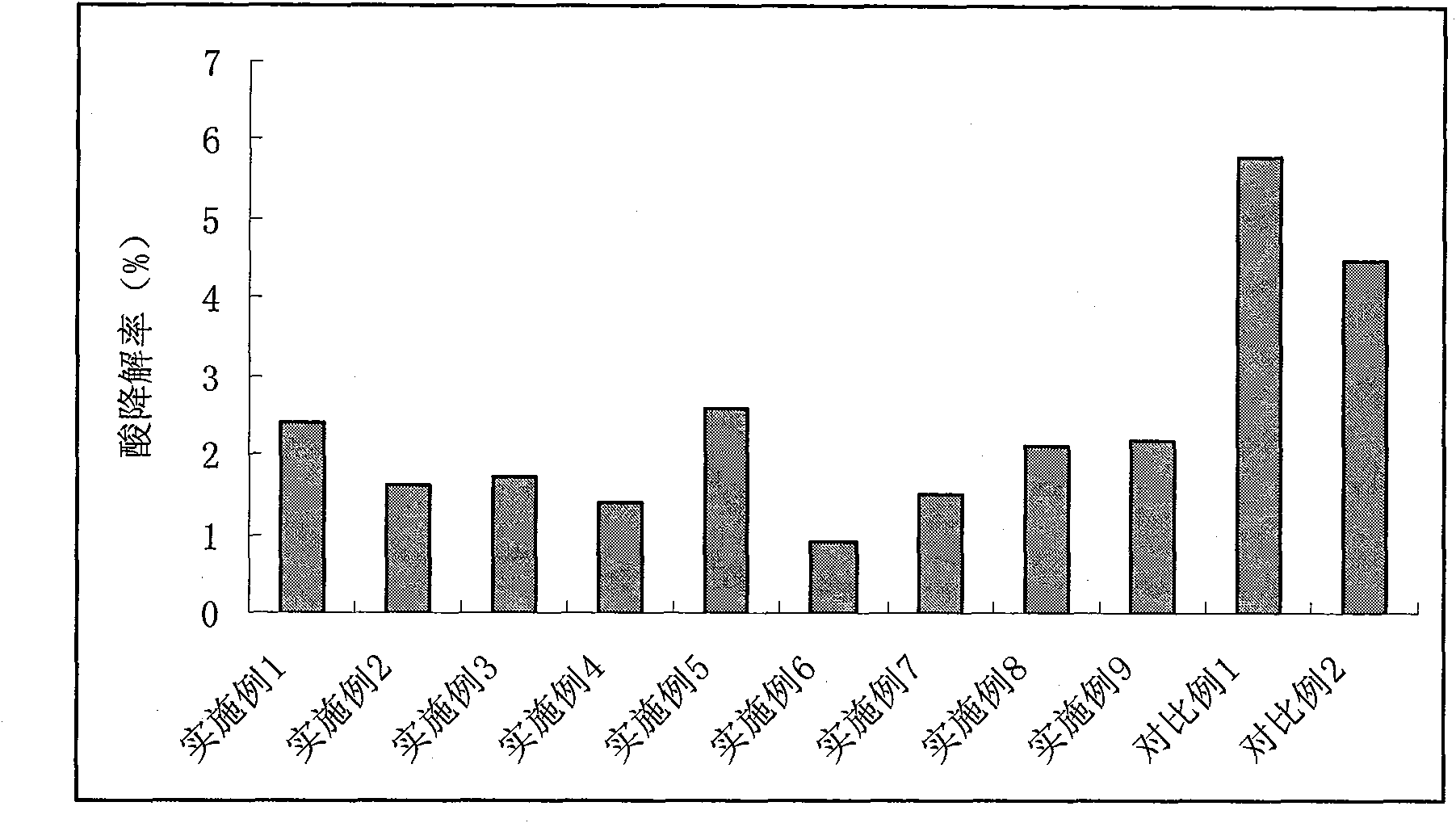
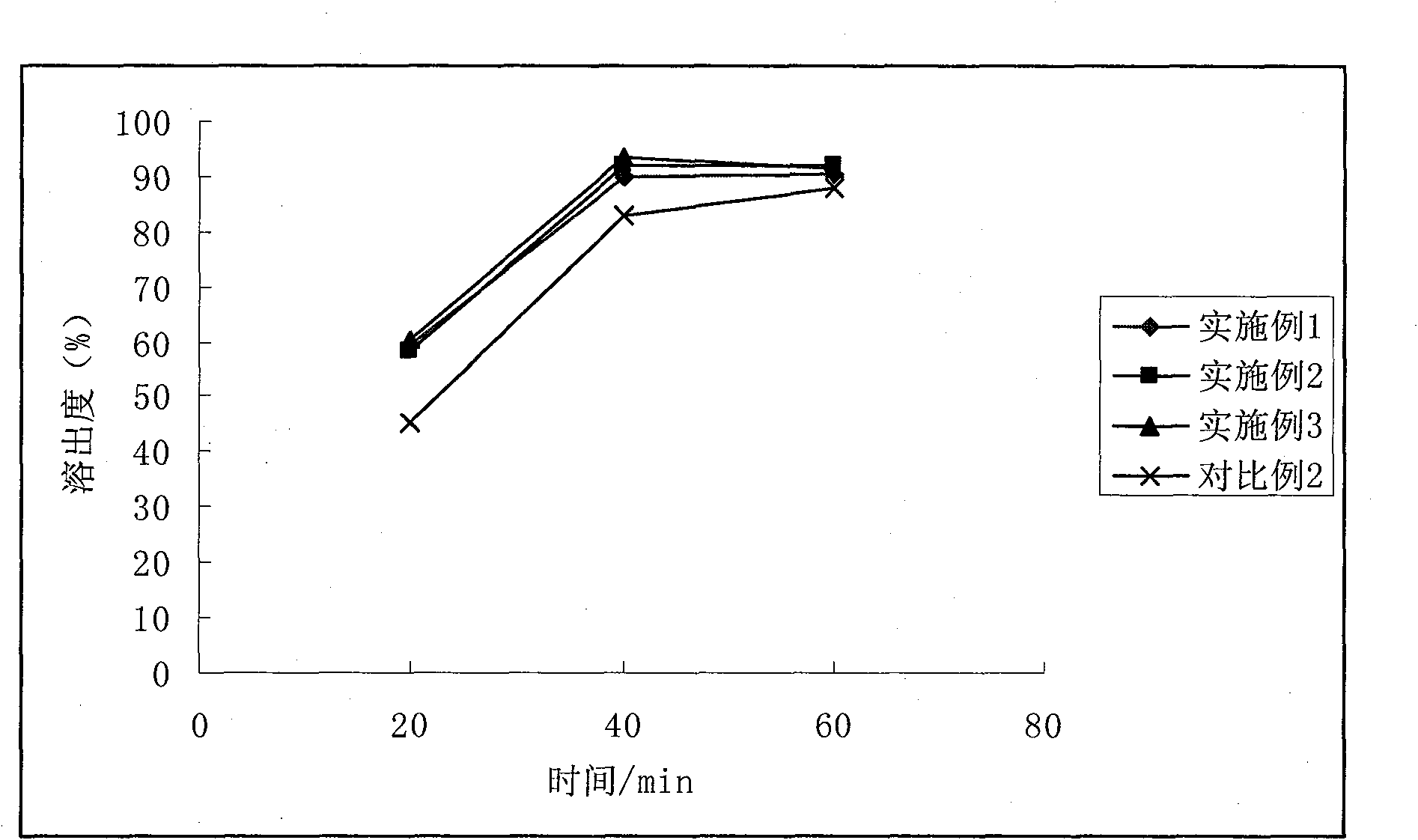
Esomeprazole magnesium enteric-coated pellet and preparation method thereof
ActiveCN102670521AProtect active ingredientsFix stability issuesOrganic active ingredientsDigestive systemMedicineIsolation layer
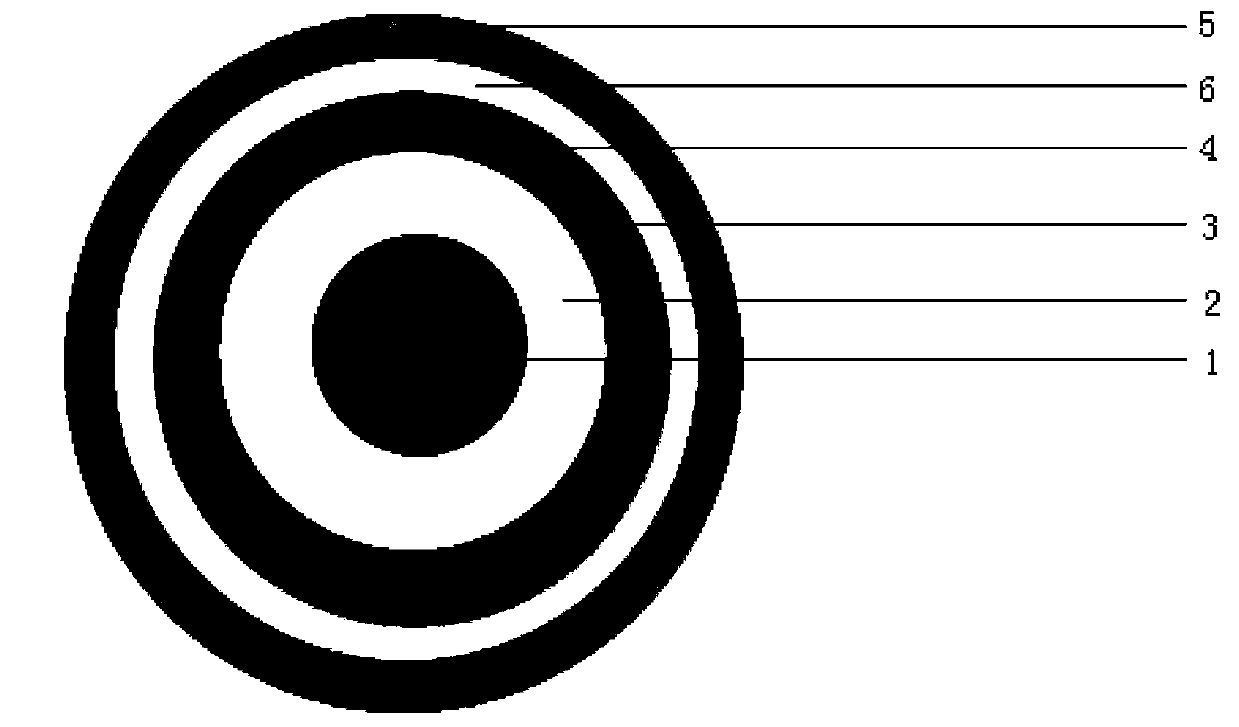
The invention belongs to the field of pharmacy and relates to a medicinal preparation taking esomeprazole magnesium as an active ingredient, in particular to an esomeprazole magnesium enteric-coated pellet and a preparation method thereof. According to the esomeprazole magnesium enteric-coated pellet, medicines can be rapidly released in intestinal tracts. The esomeprazole magnesium enteric-coated pellet comprises the following structural layers in sequence from inside to outside: a medicine-contained layer, an isolation layer and an enteric-coated layer.
Owner:珠海润都制药股份有限公司
Enteric-coated pellet preparation of proton pump inhibitor and preparation method thereof
ActiveCN102119927AEvenly dispersedAvoid degradationOrganic active ingredientsDigestive systemWater solubleBioavailability
The invention relate to an enteric-coated pellet preparation of proton pump inhibitor and a preparation method thereof. The enteric-coated pellet preparation of proton pump inhibitor provided by the invention is composed of a blank pellet core, a drug-loaded layer, isolating layers (I) and (II) and an enteric-coated layer, wherein both the drug-loaded layer and the isolating layer (I) contain water soluble inorganic bases, and the base used in the drug-loaded layer contains sodium hydroxide and another water soluble inorganic base which can form buffer with sodium hydroxide in a water solution and the pH of which is in an alkaline environment of 11-12 (excluding 11). The enteric-coated pellet of proton pump inhibitor provided by the invention successfully solves the technical problems of the existing enteric-coated pellet preparation of proton pump inhibitor, and is a superior preparation with high drug loading efficiency, good anti-acid effect, high release rate, good repeatability, high bioavailability and good stability.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Pantoprazole sodium enteric-coated pellet
ActiveCN101596165AImprove stabilityAbsorb evenlyOrganic active ingredientsDigestive systemBioavailabilityPantoprazole Sodium
The invention discloses a pantoprazole sodium enteric-coated pellet which contains the following components in percentage by weight from inside to outside: 20-60 percent of blank core pellet, 4-38 percent of medicinal layer containing pantoprazole sodium and one or more medicinal excipients, 2-15 percent of isolated layer and 15-26 percent of enteric-coated layer. The invention also discloses a preparation method and an application of the enteric-coated pantoprazole sodium pellet. The pantoprazole sodium enteric-coated capsule has the advantages of better stability, uniform absorption, smaller difference of bioavailability among individuals, and the like.
Owner:Yung Shin Pharm Ind (Kunshan) Co Ltd
Omeprazole composition and preparing process thereof
ActiveCN101120930AOvercome acidic degradationPH value is stableOrganic active ingredientsDigestive systemSodium bicarbonateMedicine
A compound of the omeprazole and the Sodium Bicarbonate comprise a plurality of enteric-coated pellets composed by the omeprazole and the Sodium Bicarbonate, the capsules composed by Sodium Bicarbonate of any mode. The enteric-coated pellets comprise a pellet core, an isolation layer and an enteric-coated layer. The present invention has the advantages that acid degradation of the omeprazole is resolved by the enteric-coated layer, which prolongs binding between the gastric wall and omeprazole. When Sodium Bicarbonate of any mode neutralizing the gastric acid reaches a certain PH value, the enteric-coated layer can disintegrate and omeprazole can be released. PH value in the stomach remains stable during the releasing process. Another advantage of the present invention is that the compound can be taken at any time when stomachache happens.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Omeprazole enteric coated pellets formulation and preparation method
The invention discloses an omeprazole enteric micropill preparation and a preparation method thereof. The preparation takes alkali salt containing omeprazole or a single antimer of the omeprazole as an active pill core, and contains a film isolating and coating layer and an enteric coating layer. The preparation takes zein as an isolating and coating material, thereby improving the stability of micropills. The preparation method uses alcohol as menstruum, thereby further improving the stability of the omeprazole micropills.
Owner:SHOUGUANG FUKANG PHARMA
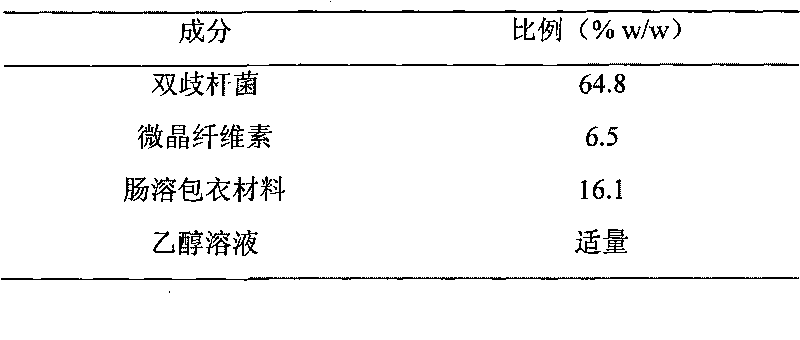
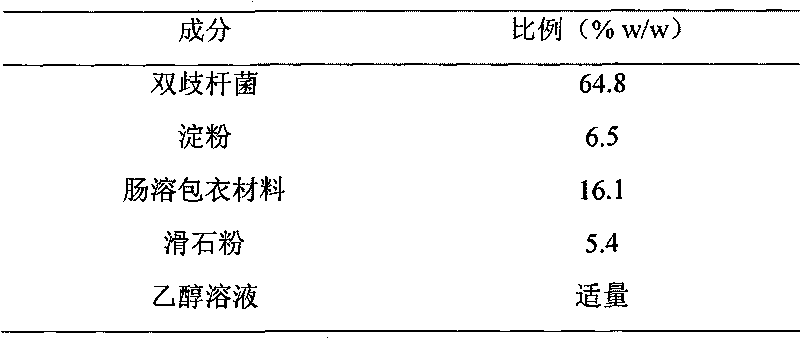
Probiotic pellet preparation and preparing method thereof
InactiveCN101757042APlay a role in disease prevention and treatmentBacteria material medical ingredientsDigestive systemCurative effectSolvent
The invention discloses a probiotic pellet preparation and a preparing method thereof. The preparation is a pellet which consists of a probiotic pellet core and an enteric coating layer wrapping the probiotic pillet core. The preparing method comprises the following steps: (1) evenly mixing probiotics and excipients, adding solvents and stirring the mixture to prepare wet material; (2) extruding, cutting and rounding the wet material prepared in step (1) to prepare a spherical wet granule and drying and selecting the probiotic pellet core; and (3) spraying an enteric coating material solution to the probiotic pellet core and drying to obtain an enteric coated pellet core. The probiotic pellet preparation prepared in the method has high intestinal colonization rate and reliable curative effect after being taken orally.
Owner:BEIJING TRADE STAR MEDICAL TECH
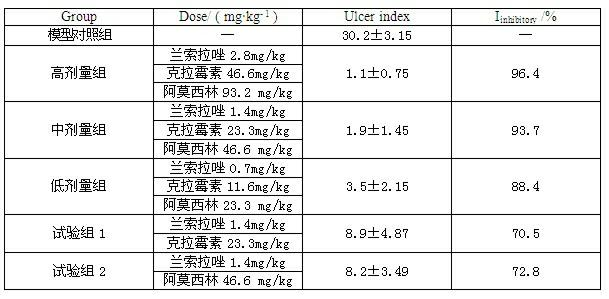
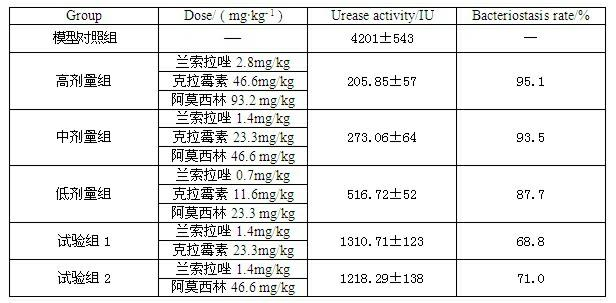
Compound capsule and preparation method thereof
InactiveCN102091084AQuick effectReduce adverse reactionsAntibacterial agentsDigestive systemLansoprazolePeptic ulcer
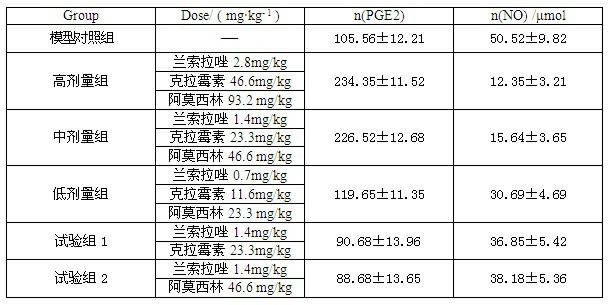
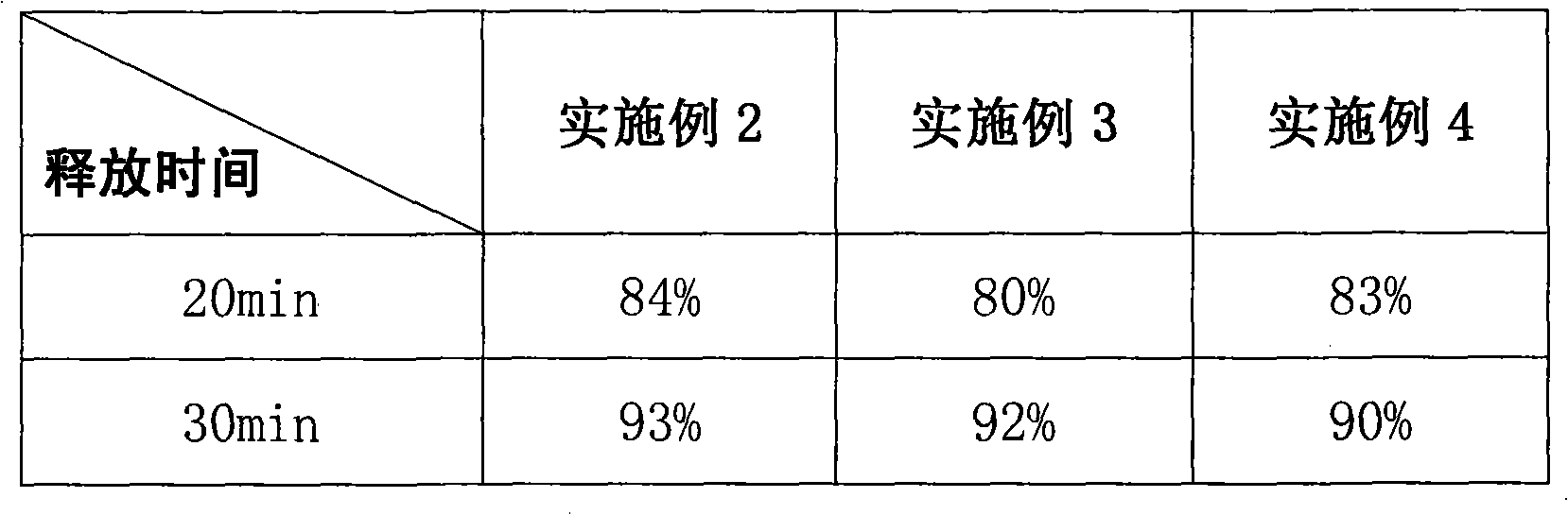
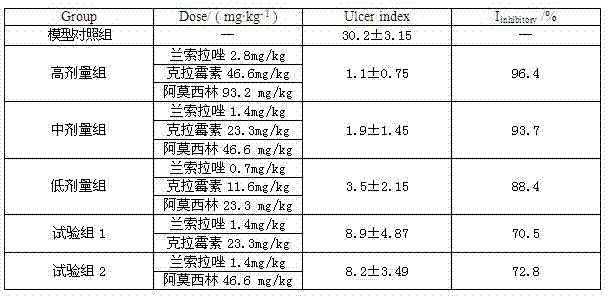
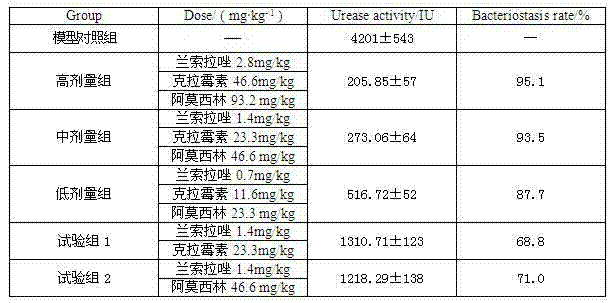
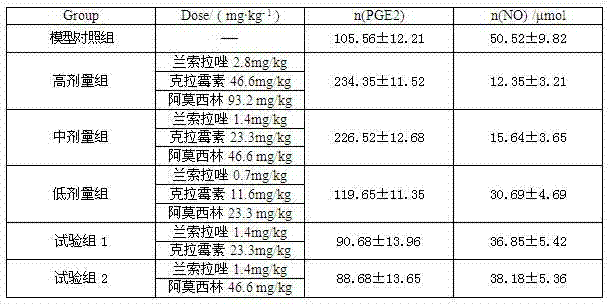
The invention relates to a compound capsule and a preparation method thereof. The capsule comprises lansoprazole enteric coated pellets, clarithromycin stomach-soluble pellets and amoxicillin stomach-soluble pellets. The compound capsule is administered twice one day based on the following dose each time: lansoprazole 20-40 mg, clarithromycin 400-600 mg, and amoxicillin 900-1100 mg. The capsule provided by the invention has a distinct effect on peptic ulcer, is used for thoroughly killing helicobacter pylori, and has the advantages of quick action, strong effect, improved bioavailability, convenience in administration and low cost.
Owner:王勇
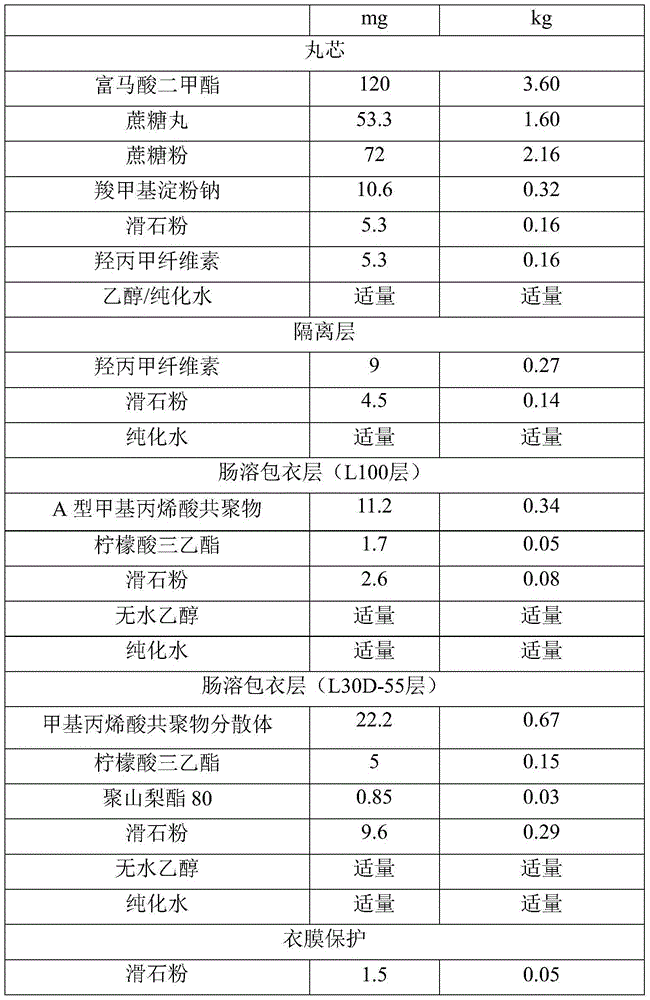
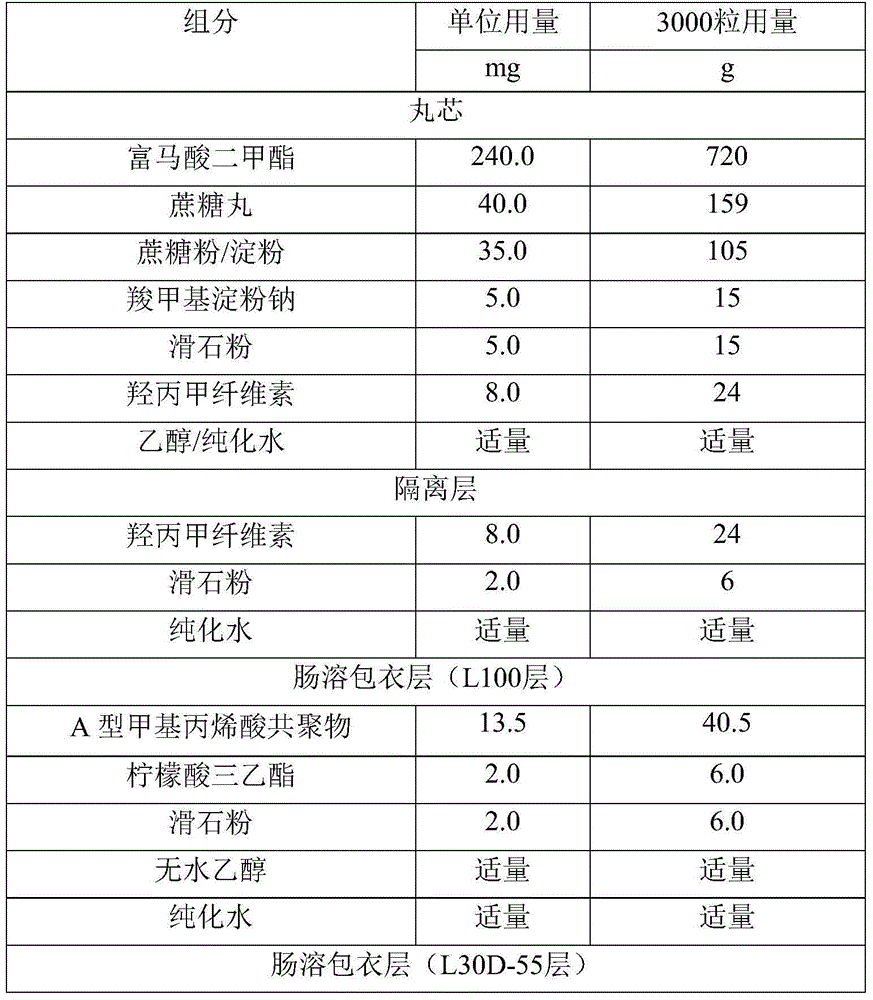
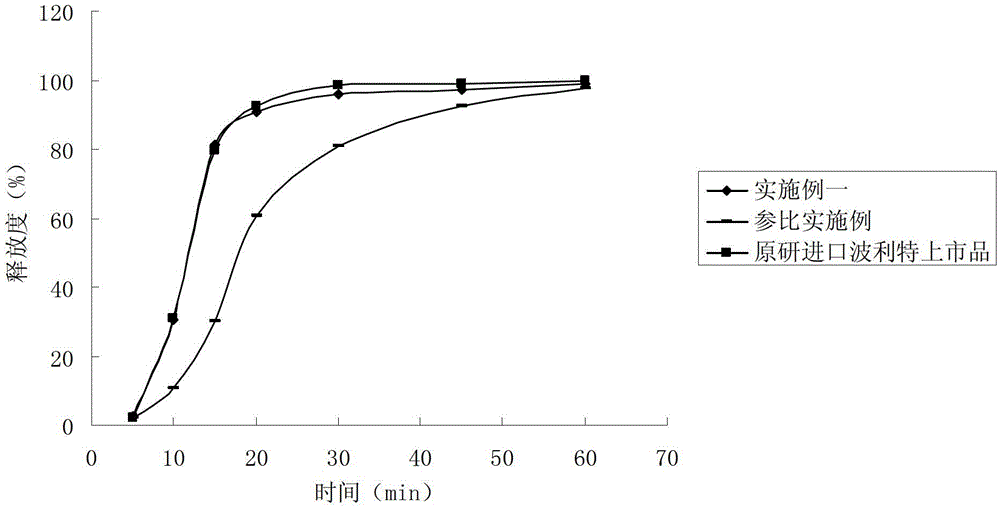
Dimethyl fumarate enteric-coated pellets and preparation method thereof
ActiveCN104971048AReduce stimulationPromotes even distributionOrganic active ingredientsGranular deliveryMedicineEnteric coated pellets
The invention discloses dimethyl fumarate enteric-coated pellets and a preparation method thereof. Each enteric-coated pellet comprises a blank pellet core, an active drug layer wrapping the blank pellet core, an isolating layer, an inner layer enteric-coated coating layer and an outer layer enteric-coated coating layer from inside to outside. The enteric-coated pellets are simple in process, complete in release, good in reproducibility, small in gastrointestinal tract irritation, safe and effective.
Owner:SHANGHAI HUILUN BIOLOGICAL TECH CO LTD
Rabeprazole sodium enteric-coated pellet and preparation method thereof
ActiveCN103599087AGood compatibilityImprove securityOrganic active ingredientsDigestive systemInsulation layerAdhesive
A rabeprazole sodium enteric-coated pellet is composed of a pellet core, an insulation layer and an enteric-coated layer from the inside to the outside, wherein by the total weight of the enteric-coated pellet, the content of the pellet core is 50 to 70 wt%, the content of the insulation layer is 10 to 20 wt%, and the content of the enteric-coated layer is 20 to 40 wt%; the pellet core comprises rabeprazole, a filling agent, an adhesive, a disintegrating agent, and an alkalizer, by the total weight of the pellet core, the content of the rabeprazole is 7 to 9 wt%, the content of the filling agent is 60 to 75 wt%, the content of the adhesive is 5 to 10 wt%, the disintegrating agent is 4 to 8 wt%, and the content of the alkalizer is 5 to 12 wt%. The rabeprazole sodium enteric-coated pellet has the advantages of low cost, stable quality, and good safety.
Owner:HAINAN HAILI PHARMACEUTICAL CO LTD
Tilmicosin enteric-coated pellets and preparation method thereof
ActiveCN105125524AGood acid stabilityGood masking of bitternessAntibacterial agentsOrganic active ingredientsBitter tasteEnteric coated pellets
The invention relates to tilmicosin enteric-coated pellets. Each tilmicosin enteric-coated pellet is composed of a pellet core and a coating layer coating the pellet core. Each pellet core is composed of, by weight, 20-60% of tilmicosin, 5-35% of poloxamer 188 and 20-75% of microcrystalline cellulose. The size of the pellet cores is not larger than the mesh aperture of a 24-mesh sieve and not smaller than the mesh aperture of a 28-mesh sieve. The weight of the coating layers accounts for 8-20% of the total weight of the tilmicosin enteric-coated pellets. The invention further relates to a preparation method of the tilmicosin enteric-coated pellets. The tilmicosin enteric-coated pellets can cover the bitter taste of the tilmicosin, the acid stability of the preparation is ensured, and the biological utilization degree is remarkably improved.
Owner:CHINANIMAL NANJING VETERINARY DRUGS
Solid composition comprising a proton pump inhibitor
The present invention related to a method for oral administration of a solid composition comprising an acid labile proton pump inhibitor compound in the form of a multiple of enteric coating layered pellets, wherein the pellets are in admixture with one or more pharmaceutically acceptable thickeners capable of forming a viscous medium when dispersed in an aqueous carrier. Alternatively, the enteric coated pellets are in admixture with a viscous medium. The formed aqueous viscous suspension is administered through a gastric tube. The method and composition are especially aimed for treatment of patients in need of a proton pump inhibitor and having difficulties to swallow or for pediatric patients.
Owner:ASTRAZENECA AB
Ilaprazole enteric coated tablet and preparation method thereof
InactiveCN102552190AImprove acid resistanceWidely distributedOrganic active ingredientsDigestive systemOrganic solventIlaprazole
The invention provides an ilaprazole enteric coated tablet and a preparation method thereof. The ilaprazole enteric coated tablet comprises enteric coated pellets and a pharmaceutically-acceptable tablet auxiliary material, wherein each enteric coated pellet comprises a pellet core, an isolating layer and an enteric coated layer; and the pellet core comprises ilaprazole or a pharmaceutically-acceptable salt thereof and a stabilizing agent. The enteric coated pellet tablet made from ilaprazole has high acid resistance; in a prescription, barrier substances such as an acid resisting agent, a surfactant, an organic solvent, a hydrophobic substance and the like are not contained, so that the health and safety of a human body are better facilitated; in the preparation method, an organic solvent is not used, so that operation is easy, and active substances are released quickly and stably; and moreover, the pellets in the tablet can be widely and uniformly distributed into an intestinal tract after administration, a dosage is poured out in a scatter way, and the distribution area of a medicament on the surface of the intestinal tract is increased, so that the stimulation of the medicament on the intestinal tract can be reduced or eliminated, and the bioavailability of the medicament is enhanced.
Owner:LIVZON PHARM GRP INC
Doxycycline hyclate enteric-coated pellet
ActiveCN101596162AAvoid local irritationAvoid adverse reactionsPowder deliveryTetracycline active ingredientsPeristalsisMedicine
The invention discloses a doxycycline hyclate enteric-coated pellet which comprises the following components in percentage by weight from inside to outside: 48-58 blank core pellet, 35-40 medicinal layer and 4-13 enteric-coated layer. The invention also discloses a method for preparing the doxycycline hyclate enteric-coated pellet. The doxycycline hyclate enteric-coated pellets prepared by the method are filled into a capsule, and an obtained enteric-coated preparation has the advantages of increased contact area with the intestinal tract, high bioavailability, good stability, steady release in vitro and vivo, no influence by gastrointestinal peristalsis and food intake, uniform absorption, smaller difference of bioavailability among individuals, and the like.
Owner:德全药品(江苏)股份有限公司
Esomeprazole enteric-coated tablets and preparation method thereof
The invention discloses esomeprazole enteric-coated tablets and a preparation method thereof. The formula of the tables comprises esomeprazole magnesium, and an inert pellet core, a binder, a dispersant and a disintegrant which are used for tablet preparation. The preparation method comprises spraying and coating esomeprazole magnesium on the blank pellet core, coating an isolating layer and an enteric coated layer, making into enteric-coated pellets, and making into the required enteric-coated preparation by using the specific tablet adjuvants and tabletting method. The esomeprazole enteric-coated tablets and the preparation method thereof disclosed by the invention have the characteristics that by adopting specific tablet preparation method, the shortcoming that the enteric coated layers of the enteric-coated pellets are easy to be broken during tabletting process to affect the acid resistance of esomeprazole enteric-coated tablets is effectively overcome.
Owner:北京华禧联合科技发展有限公司
Proton pump inhibitor enteric coated pellet and preparation and preparation method thereof
ActiveCN103356489AStable in natureQuality improvementOrganic active ingredientsDigestive systemAnti-Adhesion AgentPlasticizer
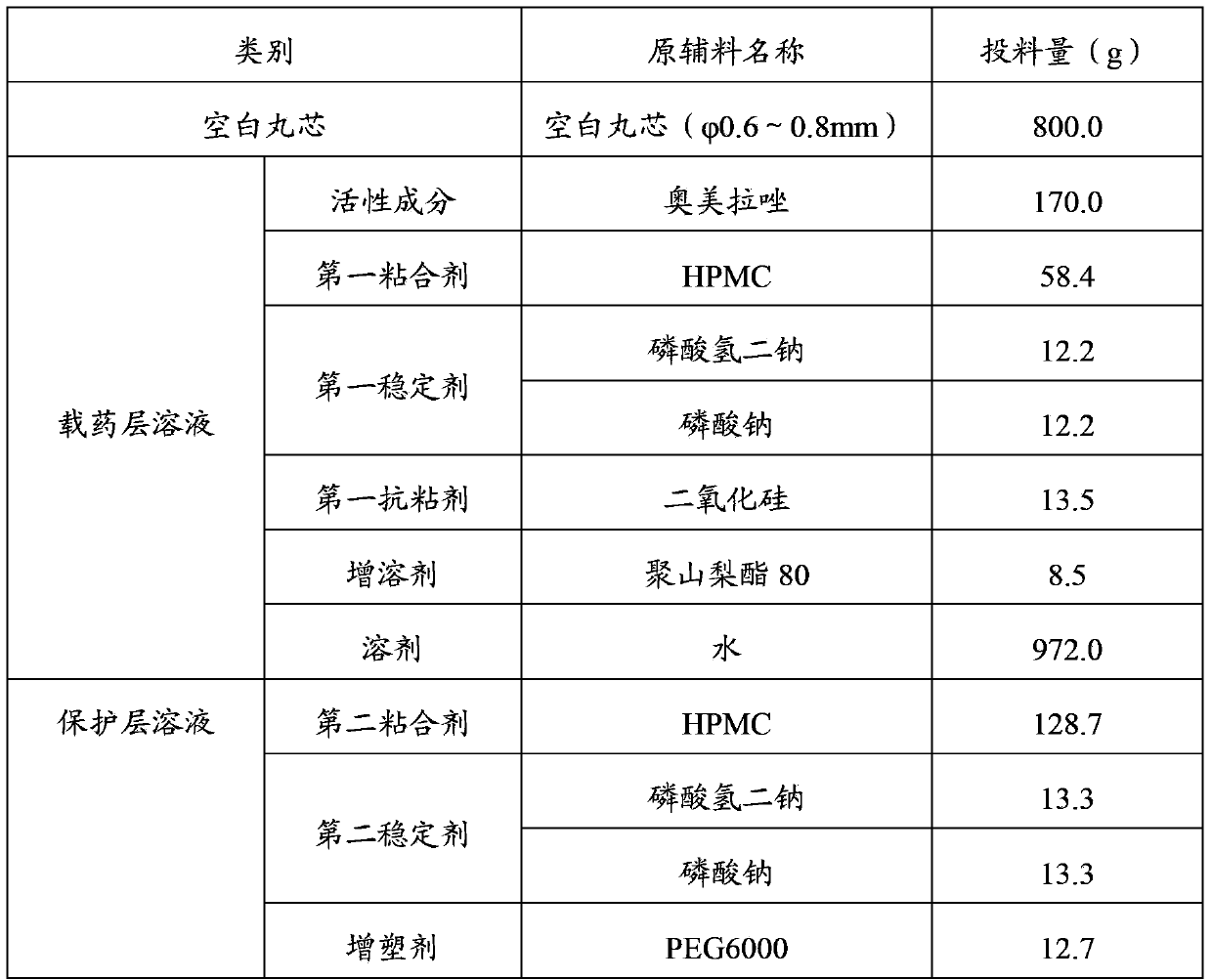
The invention relates to the field of medicines, especially to a proton pump inhibitor enteric coated pellet and a preparation and a preparation method thereof. The proton pump inhibitor enteric coated pellet is composed of a blank pellet core, a drug loaded layer, a protective layer, a separating layer, a waterproof layer and an enteric coating, wherein the waterproof layer is made from zein, the drug loaded layer is composed of a proton pump inhibitor, a first binder, a first stabilizing agent, a first antiadherent and a solubilizer or of the proton pump inhibitor, the first binder, the first stabilizing agent, a filler, a disintegrating agent and a first opacifier, the protective layer is composed of a second binder, a second stabilizing agent, a plasticizer, a second opacifier and an antifoaming agent, the separating layer is made of a coating material, and the enteric coating is composed of an enteric material, a plasticiser and a second antiadherent. The proton pump inhibitor enteric coated pellet and the preparation thereof in the invention have stable properties, reliable quality and high bioavailability and can avoid burst effects and moisture absorption.
Owner:KANGBOSHI PHARMA LIAONING
A kind of coating liquid composition of erythromycin enteric-coated pellets
ActiveCN102274191ASimple recipeReduce dosageAntibacterial agentsOrganic active ingredientsEnteric coated pelletsCoating drugs
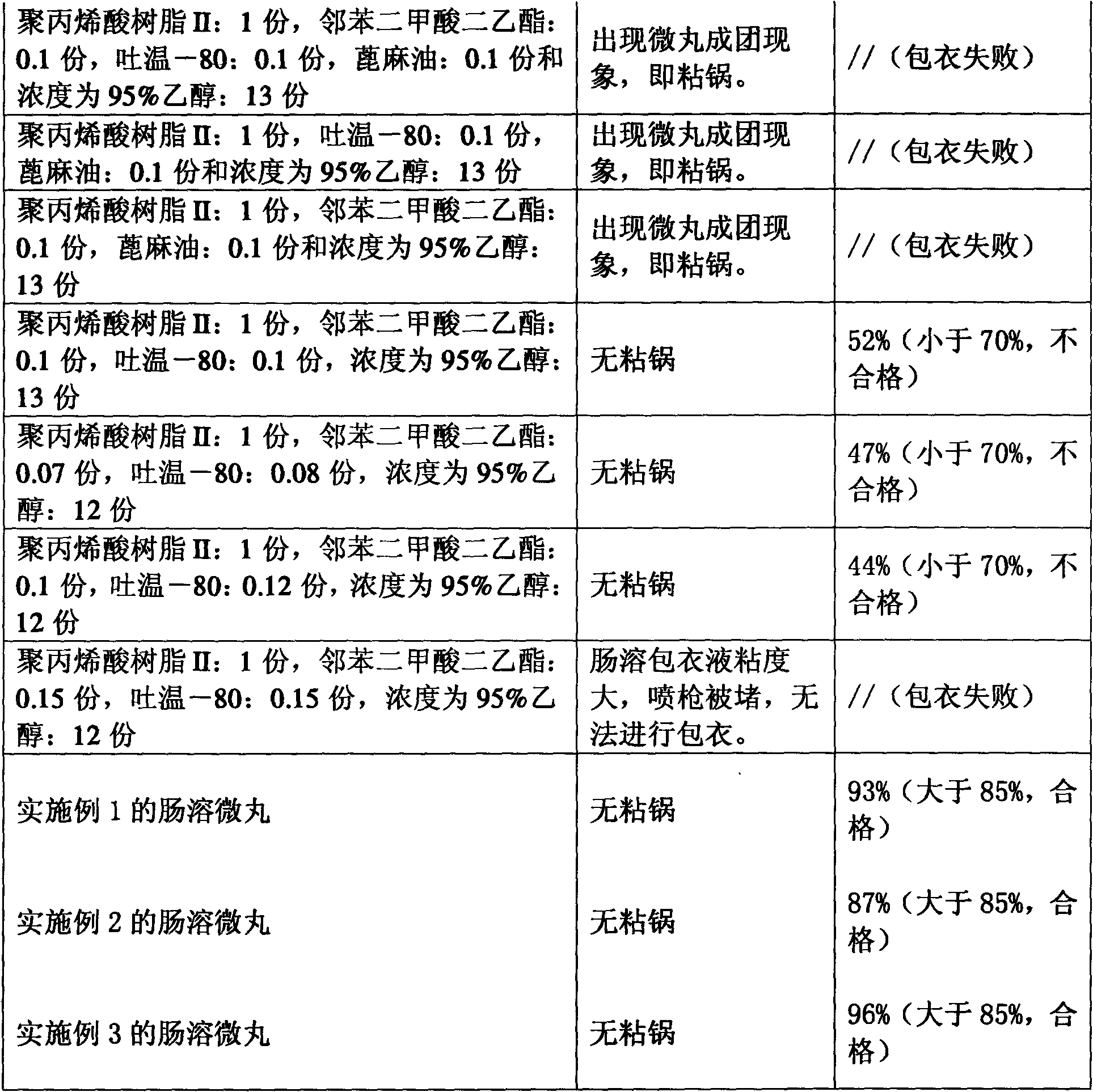
The invention provides an erythromycin enteric-coated pellet which is suitable to be prepared by a boiling coating granulator, wherein the enteric-coated coating liquid comprises polyacrylic resin II, diethyl phthalate, tween-80, and an ethanol solution. In addition, the invention also provides a process and an intermediate material for preparing the erythromycin enteric-coated pellet, such as enteric-coated coating liquid and the like.
Owner:浙江华润三九众益制药有限公司
Process for manufacture of stable oral multiple unit pharmaceutical composition containing benzimidazoles
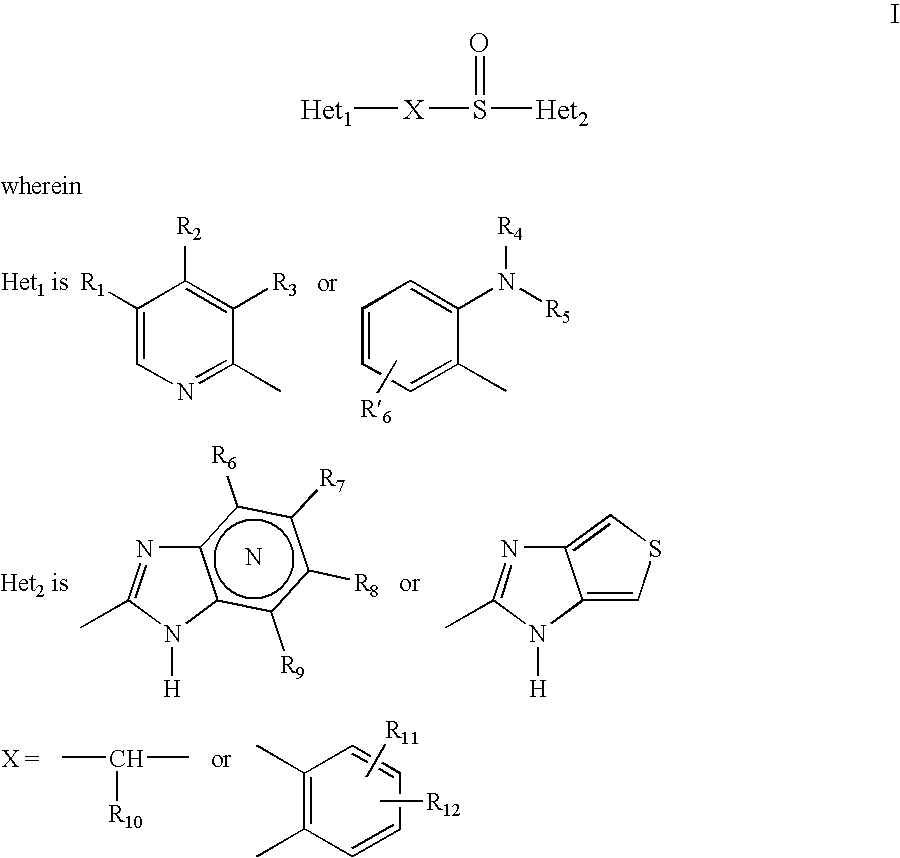
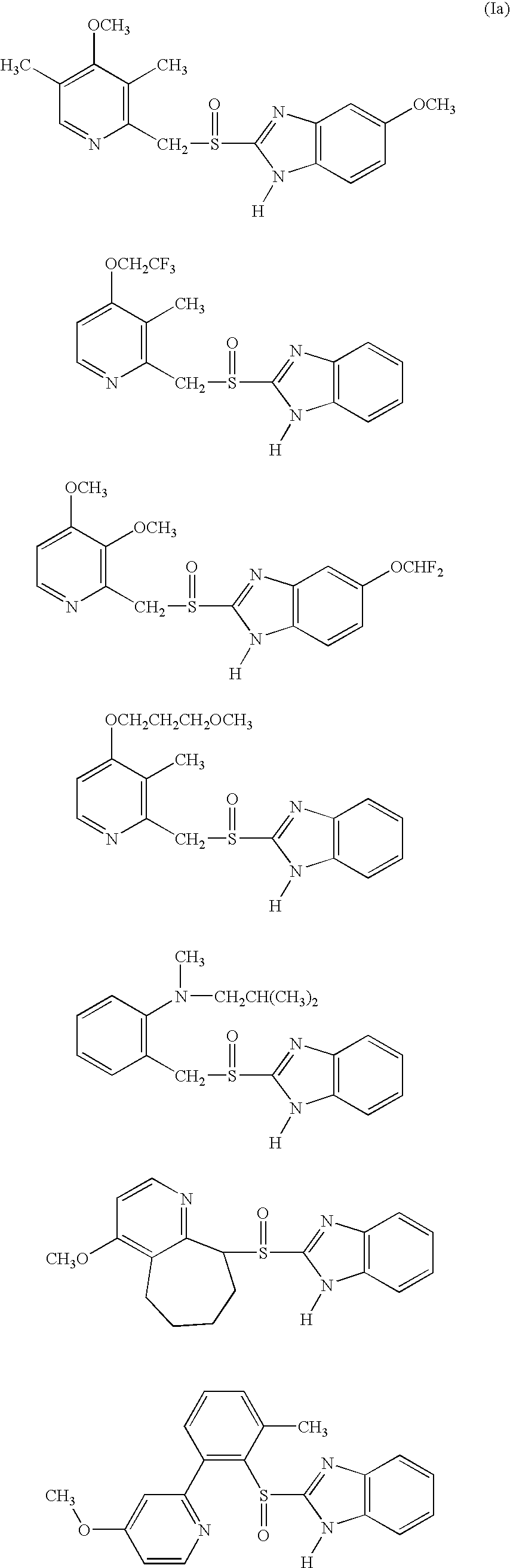
This invention relates to a process for the manufacture of a stable, oral, multiple unit pharmaceutical composition containing high concentrations of benzimidazole up to about 35 to 45% w / w, preferably up to about 40% w / w, a disintegrating agent, and one or more fillers. Surfactants in these compositions are in an enteric polymer layer and preferably not in contact with the benzimidazole. The process preferably involves sequential deposition of: (a) an alkaline material layer on non-pariel seeds to obtain treated non-pariel seeds; (b) a drug layer of the benzimidazole to obtain drug pellets; (c) a sealant polymer layer to obtain sealed pellets; and (d) an enteric polymer layer to obtain enteric-coated pellets.
Owner:THEMIS LAB PTE LTD
Process for preparing tilmicosin enteric-coated pellet by centrifuge method
ActiveCN104784123ASave spaceSimple process equipmentAntibacterial agentsOrganic active ingredientsAdhesiveBitter taste
The invention discloses a process for preparing a tilmicosin enteric-coated pellet by a centrifuge method. The tilmicosin enteric-coated pellet is prepared from the following raw materials in percentage by mass: 21-26% of tilmicosin, 10-20% of an adhesive, 23-30% of microcrystalline cellulose and 10-20% of a coating material, and is prepared through the following steps: performing centrifugal pelleting on the raw materials, and coating the pellet obtained through centrifugal pelleting. The tilmicosin enteric-coated pellet is high in encapsulation efficiency, can mask the bitter taste of tilmicosin well, is dissolved out in an intestinal environment, can be used as an enteric-coated sustained-release reagent, solves the adverse reaction caused after the tilmicosin enteric-coated pellet is absorbed in stomach, is simple in preparation process, and is suitable for industrial large-scale production, thereby having a very wide application prospect; besides, the raw materials are cheap and easy to obtain.
Owner:WEIDA HUNAN TECH
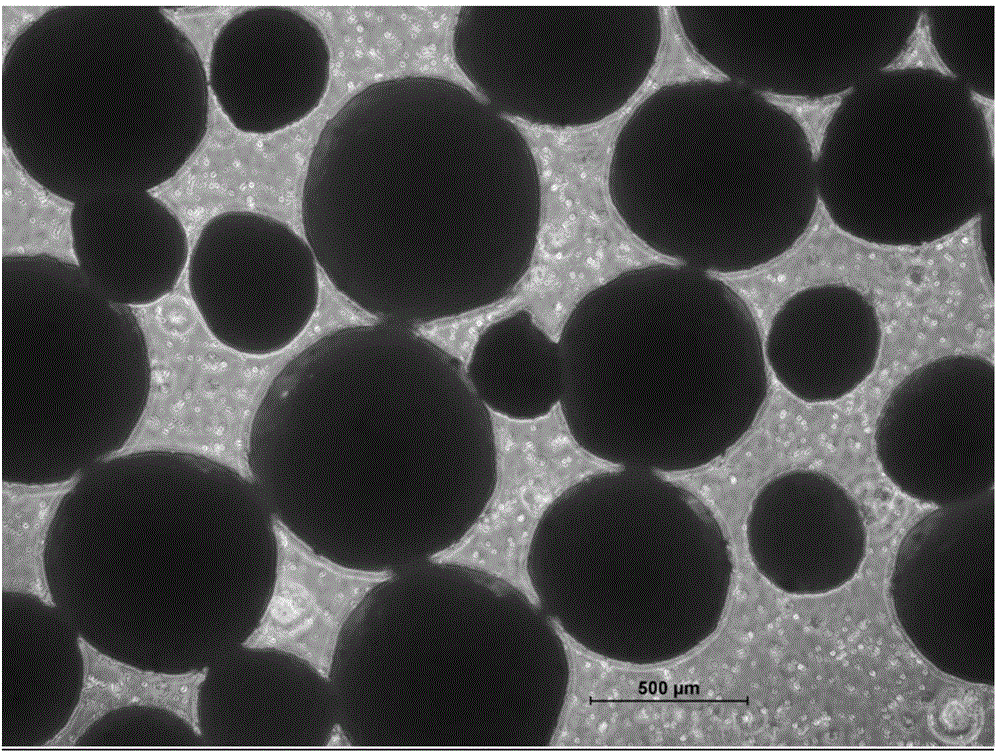
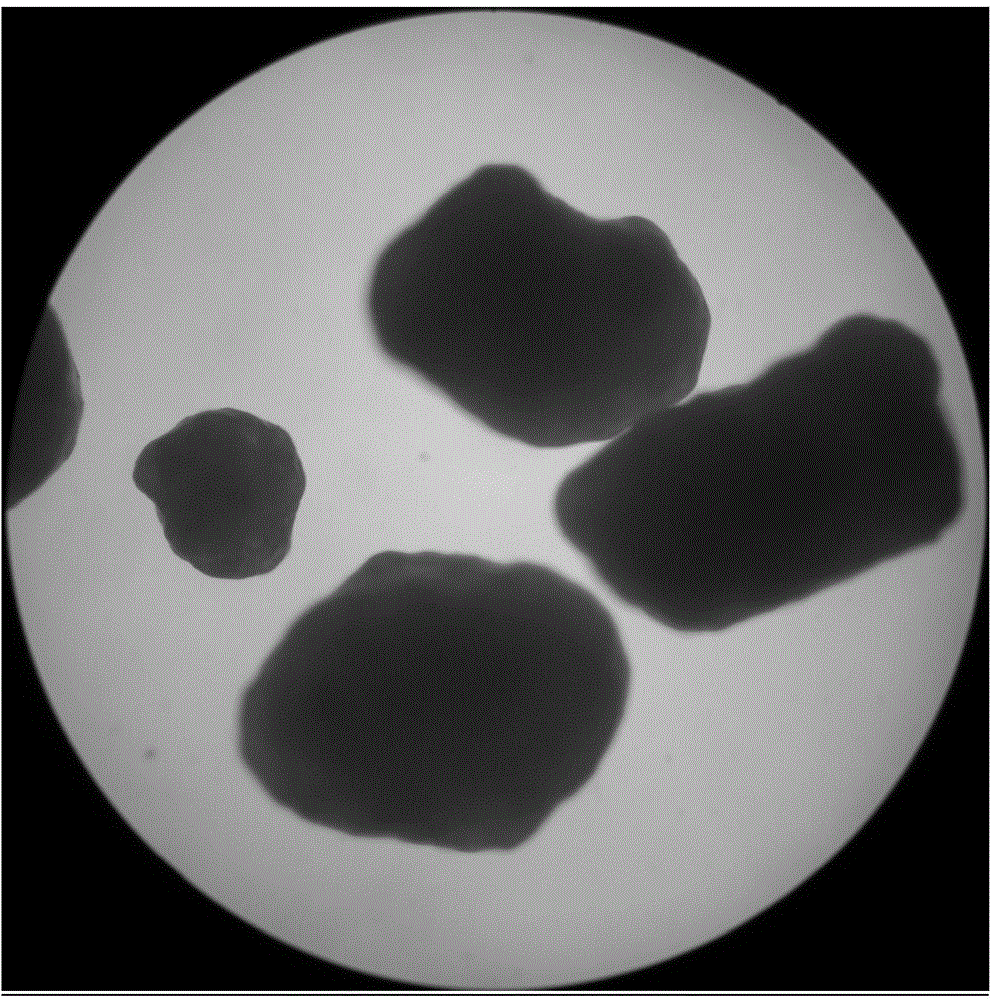
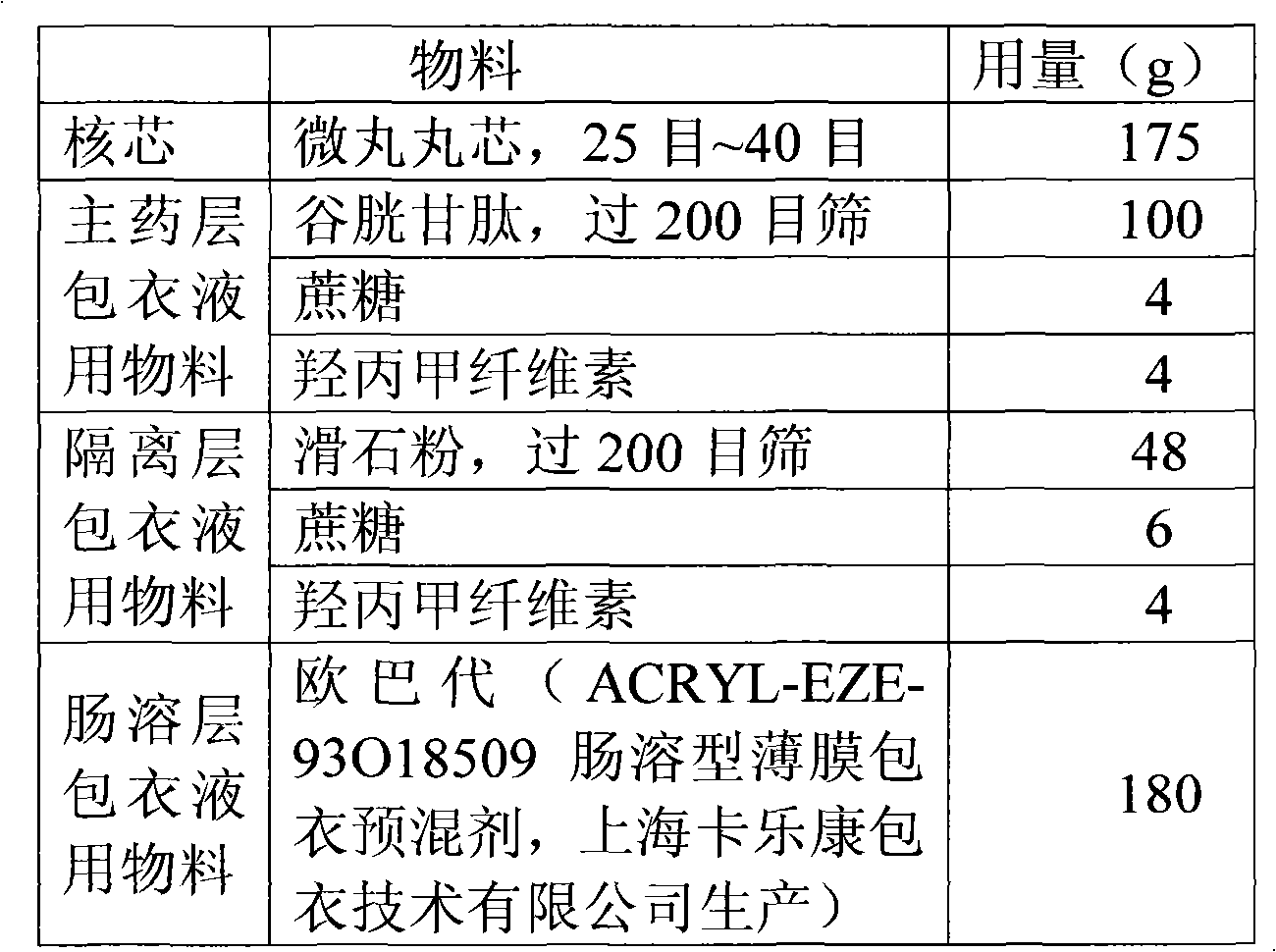
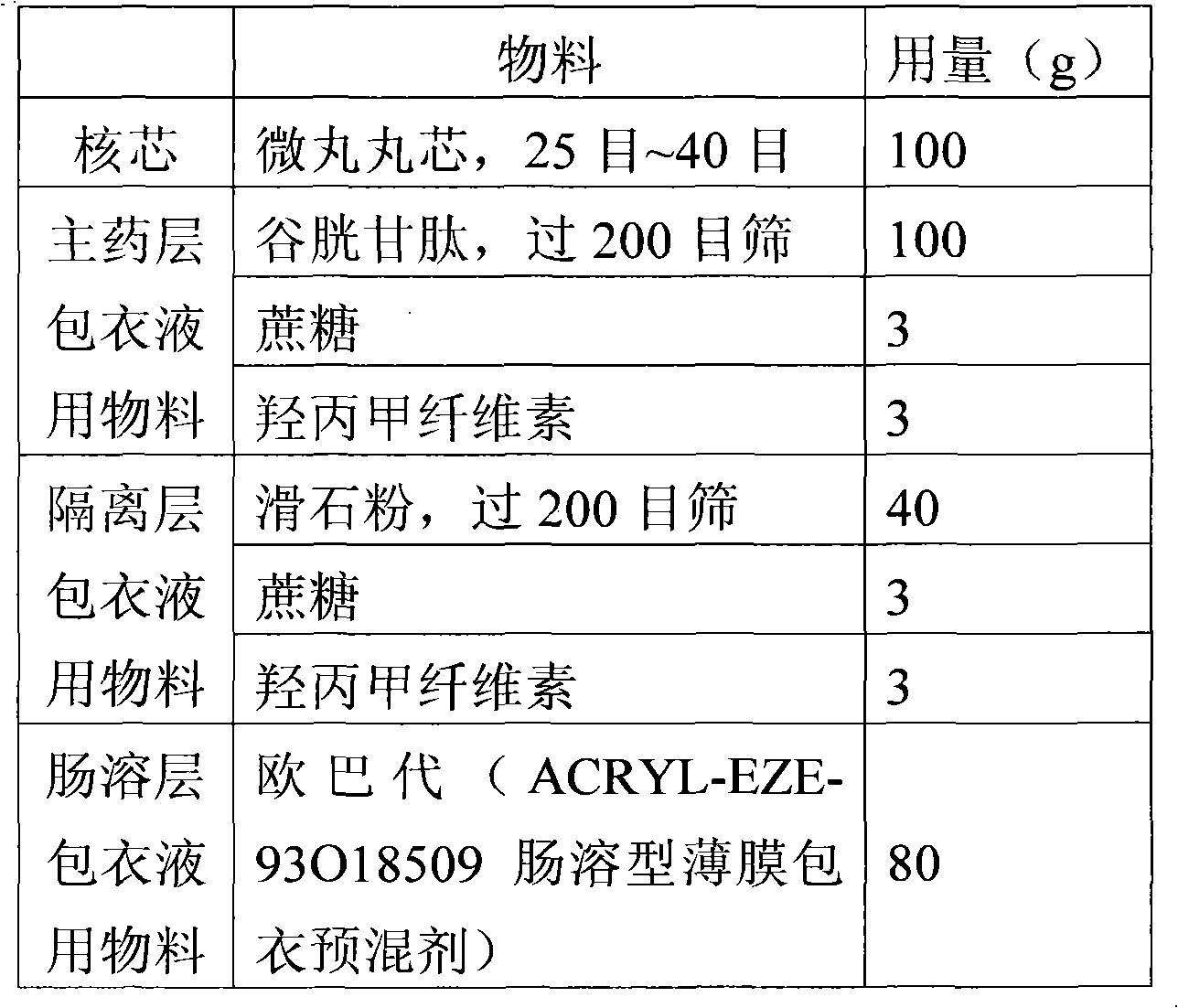
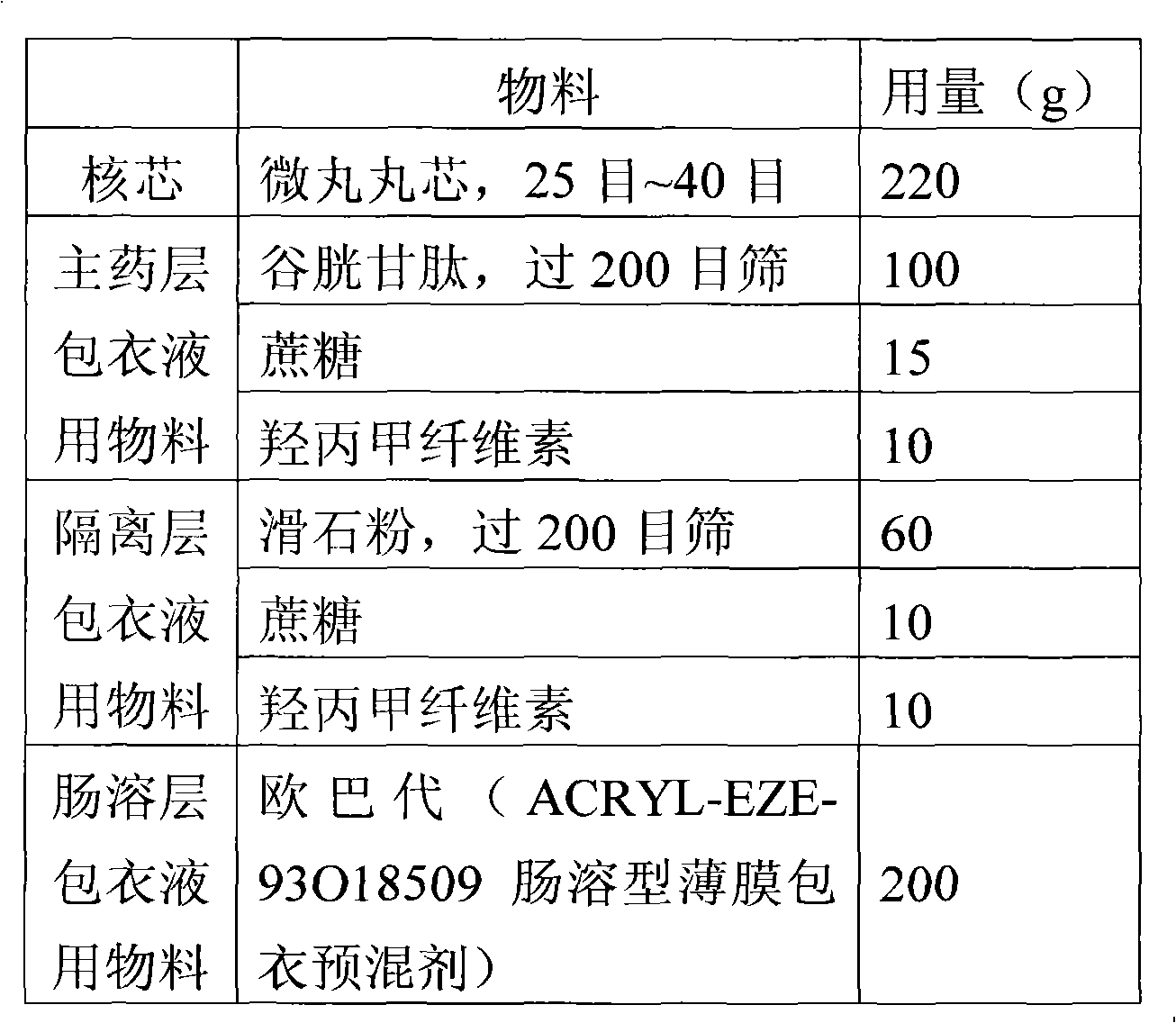
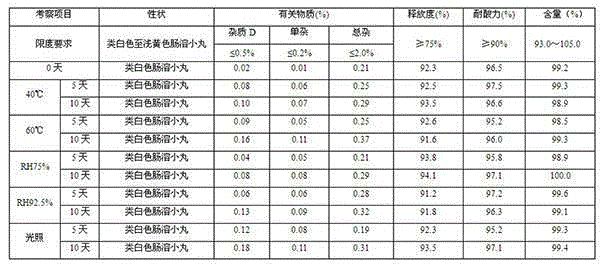
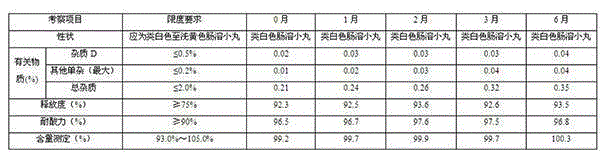
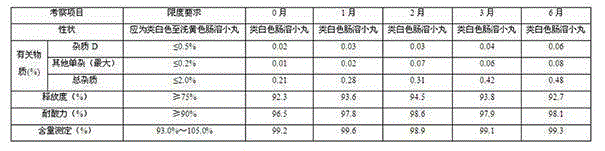
Glutathione enteric-coated pellet and preparation method thereof
InactiveCN101601659AAvoid degradationLess irritatingSenses disorderDigestive systemSmall intestineEnteric coated pellets
The invention discloses a glutathione enteric-coated pellet and preparation method thereof. The glutathione enteric-coated pellet comprises a core including glutathione and a enteric-coated layer from the inside out. Through the preparation method, main drug of the product of the invention is not released in acid and quickly released after entering into small intestine, thereby preventing the main drug from damage by gastric acid.
Owner:KUNMING JIDA PHARMA
Esomeprazole magnesium enteric capsules and preparation method thereof
ActiveCN105106168AAvoid damageIntegrity guaranteedOrganic active ingredientsDigestive systemActive agentPharmaceutical drug
The invention discloses esomeprazole magnesium enteric capsules and a preparation method thereof. Esomeprazole magnesium is prepared into enteric coated pellets, and the enteric coated pellets are filled into gastric coated capsules. Each enteric coated pellet consists of an empty pellet core, a medicine carrying layer, two isolating layers, an enteric coated layer and a film coating layer. In order to guarantee stability of medicines, alkaline materials are added in the empty pellet cores; alkali stabilizers and antioxidant are added in the medicine carrying layer; and a high-alkalinity modifier and a low-alkalinity modifier are respectively added in the two isolating layers of each enteric coated pellet. In order to guarantee a dissolution effect, surfactant is added in the enteric coated layers; and stability of products is improved owing to the film coating layers wrapping the outer layers of the enteric coated pellets. Owing to optimized formulation and technology of the pellets, smoothness of a technology is improved, work efficiency is also improved, coating time is shortened, consumption of labor and materials is reduced, and production cost is reduced.
Owner:DEZHOU DEYAO PHARMA
Lansoprazole enteric-coated pellet capsule as well as preparation method thereof
The invention relates to the field of preparations and specifically relates to a lansoprazole enteric-coated pellet capsule as well as a preparation method thereof. A technical problem to be solved in the invention is to provide a lansoprazole enteric-coated pellet capsule which is more stable and controllable in quality, and safer and more reliable for human body use. The lansoprazole enteric-coated pellet capsule comprises a pellet and a capsule shell, wherein the pellet is a pellet pill-containing core which is coated with an isolated coating layer and an enteric coating layer in sequence; the pellet pill-containing core comprises the following materials in parts by weight: 5-20 parts of lansoprazole, 30-80 parts of blank pill cores, 0.5-2 parts of a pH regulator, 1-3 parts of an adhesive and 25-45 parts of water, and a micronization particle size range of lansoprazole is preferably D95 equal to 5 mu m-8 mu m; the isolated coating layer is a mixture of a filling agent and an adhesive in a weight ratio of 1:2-1:6, or is gastric type Opadry; the enteric coating layer contains a film-forming agent and a pore-foaming agent in a weight ratio of 1:18-1:24, or is enteric type Opadry.
Owner:SICHUAN ZHIQIANG MEDICINE SCI & TECH DEV LTD
Solid dosage form comprising proton pump inhibitor and suspension made thereof
InactiveUS20060134210A1Fast outputRapid gelling timeAntibacterial agentsPowder deliveryDiseaseGastrointestinal disorder
A solid, rapidly gelling oral pharmaceutical dosage form, as well as an aqueous formulation prepared thereof, comprising a) an acid sensitive proton pump inhibitor as active ingredient distributed in a multitude of enteric coated pellets, and b) a suspension modifying granulate. Furthermore, the invention relates to an improved process for the manufacture and the use of such formulation in medical treatment, including prevention of gastrointestinal disorders in humans.
Owner:ASTRAZENECA AB
Nano zinc oxide enteric-coated pellet and preparation method thereof
InactiveCN109276561AAchieve antidiarrheal effectRealize that most of zinc oxide exerts anti-diarrheal effect in molecular form in the small intestineInorganic active ingredientsDigestive systemIsolation layerTalc / Zinc Oxide
The invention relates to a nano zinc oxide enteric-coated pellet and a preparation method thereof. Existing zinc oxide products for preventing and controlling piglet diarrhea are no longer able to meet market demands. The nano zinc oxide enteric-coated pellet sequentially comprises a zinc oxide pellet core, an isolation layer, an enteric layer and a protective layer from inside to outside. The selected zinc oxide raw material is pharmaceutical grade 99% zinc oxide which is subjected to nano treatment or ordinary feed grade zinc oxide with a purity of more than 95%, the zinc oxide particle sizeis below 3,000 mesh, and the nano zinc oxide enteric-coated pellet is prepared from the raw materials in parts by mass: 30-90 parts of the nano zinc oxide pellet core, 0-40 parts of the sum of the isolation layer and the protective layer and 10-30 parts of the enteric layer. According to the nano zinc oxide enteric-coated pellet and the preparation method thereof, excessive zinc is avoided, zincpollution is reduced, the dosage is small, zinc emissions in animal faeces are greatly reduced, soil pollution is prevented, and environmental protection is achieved.
Owner:浙江诚缘生物科技有限公司
Dexmethylphenidate hydrochloride dual-release preparation and preparation method thereof
InactiveCN101933913ASimple processIncrease productivityOrganic active ingredientsNervous disorderDual releaseSide effect
The invention belongs to the field of medicine, and in particular relates to a dexmethylphenidate hydrochloride dual-release preparation and a preparation method thereof. The preparation of the invention comprises pellets with different release performance, wherein the pellets comprise 10 to 70 percent of quick-response pellets and 20 to 60 percent of enteric-coated pellets based on the total weight of the pellets. The release mode in the preparation can achieve an ideal treatment effect and ensure that the medicament concentration in blood plasma in vivo is maintained as long as 16 to 24 hours. Compared with a dexmethylphenidate hydrochloride common preparation which is taken twice per day, the dual-release preparation can reduce a peak value of the medicament concentration in the blood plasma in vivo after the medicament is taken for the second time, and simultaneously the bioavailability of the medicament is not influenced. The dexmethylphenidate hydrochloride adopted by the invention can effectively reduce toxic and side effects brought by a racemic compound and improve a treatment effect and the medication compliance of a patient.
Owner:孙卫东
Azithromycin enteric-coated pellet capsule and preparation method thereof
InactiveCN101897670AEvenly dispersedImprove bioavailabilityAntibacterial agentsOrganic active ingredientsAzithromycinMedicine
The invention relates to an azithromycin enteric-coated pellet capsule and a preparation method thereof. The capsule comprises azithromycin mixed preparation auxiliary material which is loaded into a pellet core to form a pellet pill that is covered by isolation coating material to form an isolation layer and covered by enteric-coated material to form an enteric-coated layer. The invention azithromycin enteric-coated pellet capsule solves the problems in the background technology, can be dissolved and absorbed in intestinal tract, is rapidly and evenly dispersed in the intestinal tract after entering into the intestinal tract, and effectively improves the bioavailability of medicine. The invention also provides the preparation method of the azithromycin enteric-coated pellet capsule.
Owner:范敏华
Pancreatic enzymes enteric coated pellets and preparation
InactiveCN101279091AReasonable formulaImprove acid resistancePeptide/protein ingredientsDigestive systemDiseaseAnti-Adhesion Agent
The invention discloses a medicine preparation of trypsin enteric coated pellets. The preparation consists of active trypsin powder, a polyol medical excipient, a disintegrant and an enteric coating material containing an anti-adhesion agent. The pellet medicine preparation of the invention is prepared by an extruding-rolling technique. The preparation is used for treating dyspepsia, gastricism caused by pancreatic diseases and dyspepsia of diabetic patients, etc.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Compound capsule and preparation method thereof
InactiveCN102091084BQuick effectReduce adverse reactionsAntibacterial agentsDigestive systemLansoprazolePeptic ulcer
The invention relates to a compound capsule and a preparation method thereof. The capsule comprises lansoprazole enteric coated pellets, clarithromycin stomach-soluble pellets and amoxicillin stomach-soluble pellets. The compound capsule is administered twice one day based on the following dose each time: lansoprazole 20-40 mg, clarithromycin 400-600 mg, and amoxicillin 900-1100 mg. The capsule provided by the invention has a distinct effect on peptic ulcer, is used for thoroughly killing helicobacter pylori, and has the advantages of quick action, strong effect, improved bioavailability, convenience in administration and low cost.
Owner:王勇
Colon-targeted capsule for treating ulcerative colitis and preparation technology thereof
ActiveCN108888670ARegulatory immunityRegulate physiqueAntipyreticDigestive systemPositive controlTreatment effect
The invention relates to the technical field of medicines, in particular to a colon-targeted capsule for treating ulcerative colitis and a preparation technology thereof. Capsule contents of the colon-targeted capsule are prepared from 5-10 parts of gastric-dissolved pellets and 10-20 parts of enteric-coated pellets, the gastric-dissolved pellets are prepared from rhizoma atractylodis macrocephalae volatile oil, beta-cyclodextrin, a pill accelerator and the like, the enteric-coated pellets are prepared from coptidis rhizoma, saposhnicovia divaricata, fructus mume extracts, a pill accelerator and a colon-targeted coating material. Accordingly, the colon-targeted capsule is characterized in that the symptoms and the causes are treated, bioavailability is high, different ingredients are released in the stomach and the colon, and the capsule has the advantages that oral administration is safe and convenient, the coating prescription is simple, and industrial production can be conducted. Animal experiments show that the colon-targeted capsule has the obvious therapeutic effect on the ulcerative colitis, the treatment effect of the medium dosage is close to and even superior to the positive control drug SASP, and a brand new preparation is provided for clinically treating the ulcerative colitis.
Owner:苏州玉森新药开发有限公司
Preparation method of lumbrokinase enteric-coated pellets
InactiveCN102119928AReduce freeze-drying processEasy to producePeptide/protein ingredientsGranular deliveryFreeze-dryingAdhesive
The invention relates to a preparation method of lumbrokinase enteric-coated pellets, comprising the following steps of: washing fresh live eisenia foetida with purified water, preparing homogenate by a colloid mill, repeatedly freezing and thawing, heating, centrifuging at high speed to obtain supernatant, separating the supernatant by an anion exchange chromatographic column, eluting, concentrating, filtering to obtain a lumbrokinase concentrated solution, mixing the concentrated solution with an adhesive, spraying onto the blank pellet cores to prepare pellets, coating the pellets with enteric coatings, and screening. The raw materials of the enteric-coated pellets prepared by the method dispense with a freeze drying process, thus simplifying the production process and lowering the production cost.
Owner:JIANGZHONG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com