Dexmethylphenidate hydrochloride dual-release preparation and preparation method thereof
A double-release technology of dexmethylphenidate hydrochloride, which is applied in the fields of pharmaceutical formulation, drug delivery, and nervous system diseases, can solve the problems of drug addiction and dependence, short half-life, poor compliance, etc., and achieve simple preparation process Easy operation, less loss, stable and uniform release performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
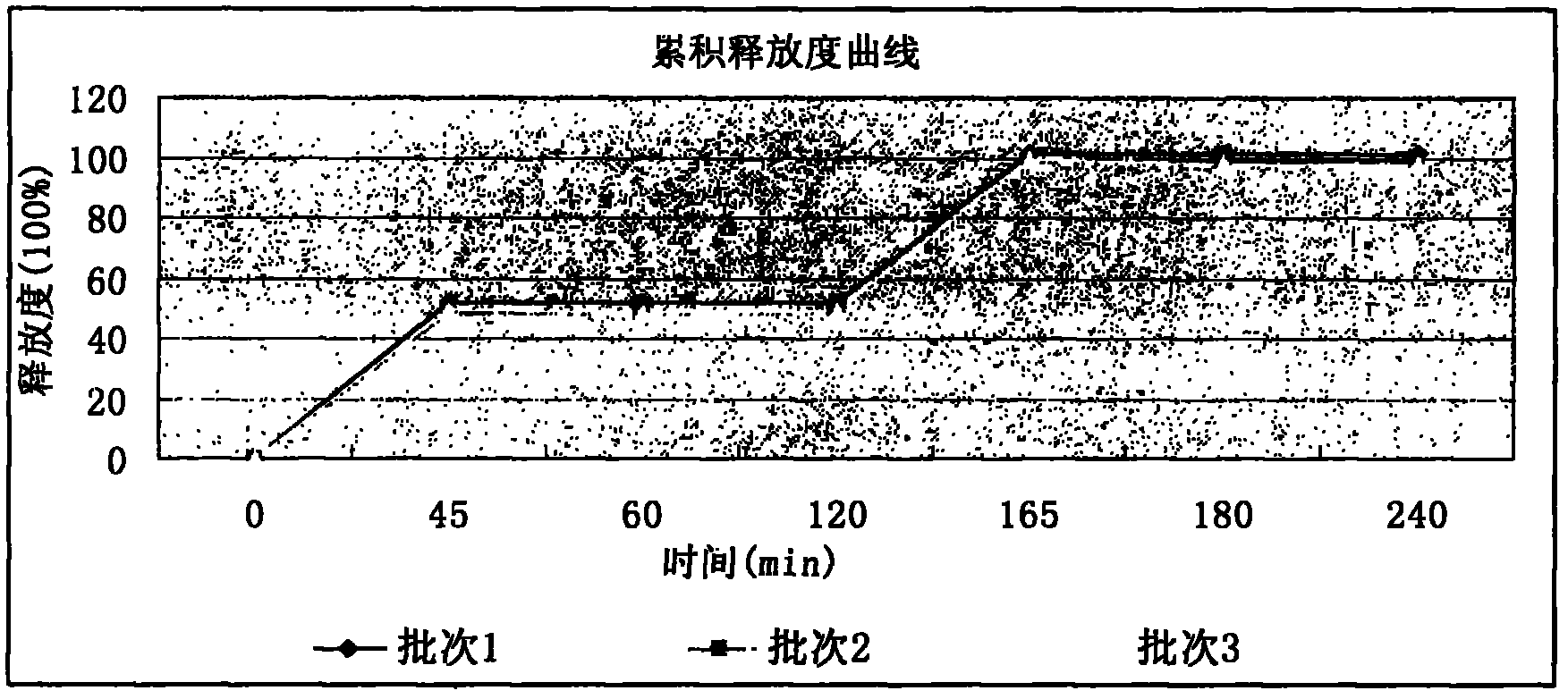
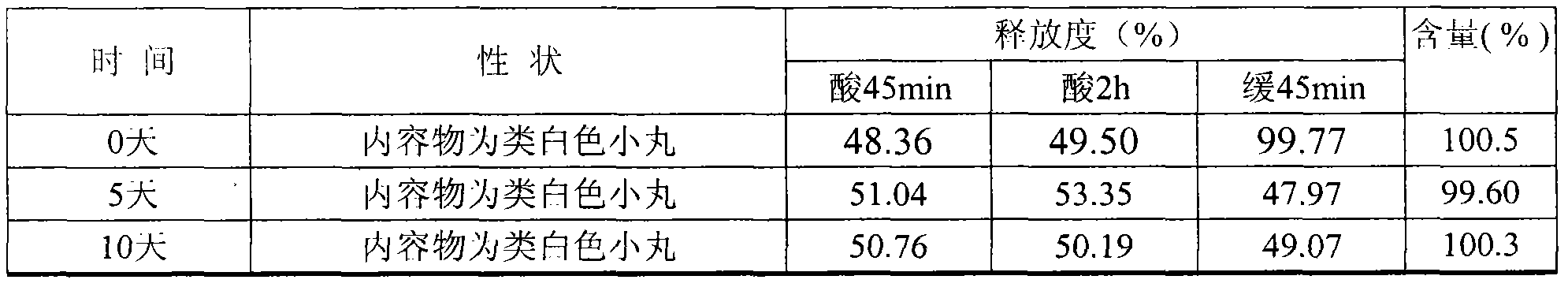
Image
Examples
Embodiment 1
[0071] prescription
[0072] (1) pellet core
[0073] Microcrystalline Cellulose 49g
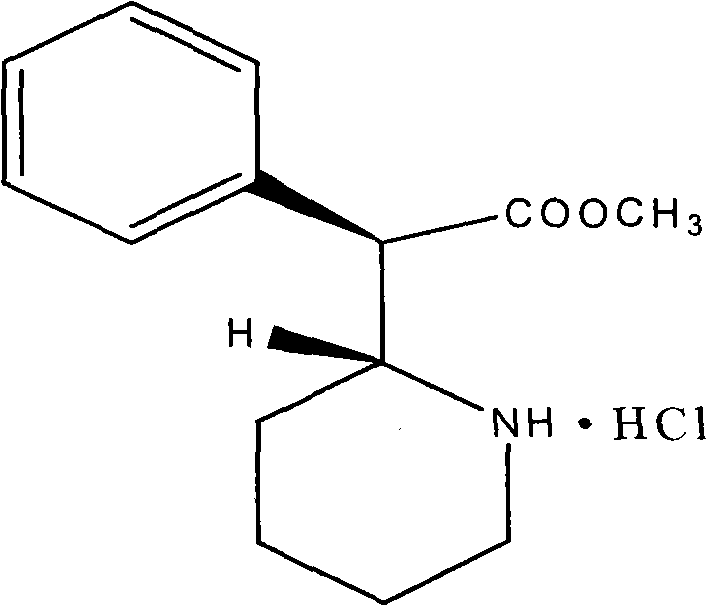
[0074] Dexmethylphenidate Hydrochloride 5.0g
[0075] Carboxymethylcellulose 1g
[0076] 2% hydroxypropyl methylcellulose solution 44ml
[0077] (2) Immediate release pellets
[0078] Pellet core 56g
[0079] 6% hydroxypropyl methylcellulose solution 11.2ml
[0080] (3) Delayed release pellets
[0081] Pellet core 100g
[0082] 15% solids aqueous dispersion of ethyl cellulose 33.3ml
[0083] Dibutyl phthalate 0.2g
[0085] Preparation process and process:
[0086] (1) Preparation process and technology of pellet core
[0087] The medicine, the filler and the disintegrant were pulverized to a certain particle size range, and after passing through an 80-mesh sieve, they were mixed in a mixer for 1 hour, and then 44ml of 2% (w / v) hydroxypropylmethylcellulose solution was added to prepare into soft material. Turn on the low-temperature refrigerator and con...
Embodiment 2
[0095] prescription
[0096] (1) pellet core
[0097] Microcrystalline Cellulose 49g
[0098] Dexmethylphenidate Hydrochloride 5.0g
[0099] Crospovidone 0.9g
[0100] 2% hydroxypropyl methylcellulose solution 44ml
[0101] (2) Immediate release pellets
[0102] Pellet core 56g
[0103] 20% Opadry I aqueous solution 11.2ml
[0104] (3) Delayed release pellets
[0105] Pellet core 250g
[0106] Udrake L30D-55 166.7g
[0107] Triethyl citrate 12.5g
[0109] water 216ml
[0110] Preparation process and process:
[0111] (1) Preparation process and technology of pellet core
[0112]The drug, filler and disintegrating agent are crushed to a certain particle size range, passed through an 80-mesh sieve, mixed in a mixer for 1 hour, and then 44ml of hydroxypropylmethylcellulose solution is added to make a soft material. Turn on the low-temperature refrigerator and control the temperature at 4-15°C. After 10 minutes, put the soft material into t...
Embodiment 3
[0120] prescription
[0121] (1) pellet core
[0122] Microcrystalline Cellulose 49g
[0123] Dexmethylphenidate Hydrochloride 5.0g
[0124] Povidone 0.5g
[0125] 2% hydroxypropyl methylcellulose solution 44ml
[0126] (2) Immediate release pellets
[0127] Pellet core 56g
[0128] 20% Opadry MP Solution 11.2ml
[0129] (3) Delayed release pellets
[0130] Pellet core 100g
[0131] 15% solids aqueous dispersion of ethyl cellulose 33.33ml
[0132] Preparation process and process:
[0133] (1) Preparation process and technology of pellet core
[0134] The drug, filler and disintegrating agent are crushed to a certain particle size range, passed through an 80-mesh sieve, mixed in a mixer for 1 hour, and then 44ml of hydroxypropylmethylcellulose solution is added to make a soft material. Turn on the low-temperature refrigerator, and control the temperature at 4-15 degrees Celsius. After 10 minutes, put the soft material into the low-temperature and high-pressure extrud...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com