Crystalline Ilaprazole sodium hydrate and preparation method thereof
A technology of ilaprazole sodium and its crystalline form, which is applied in the field of crystalline ilaprazole sodium hydrate and its preparation, achieving the effects of easy control, good storage stability, and high purity of the crystalline form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
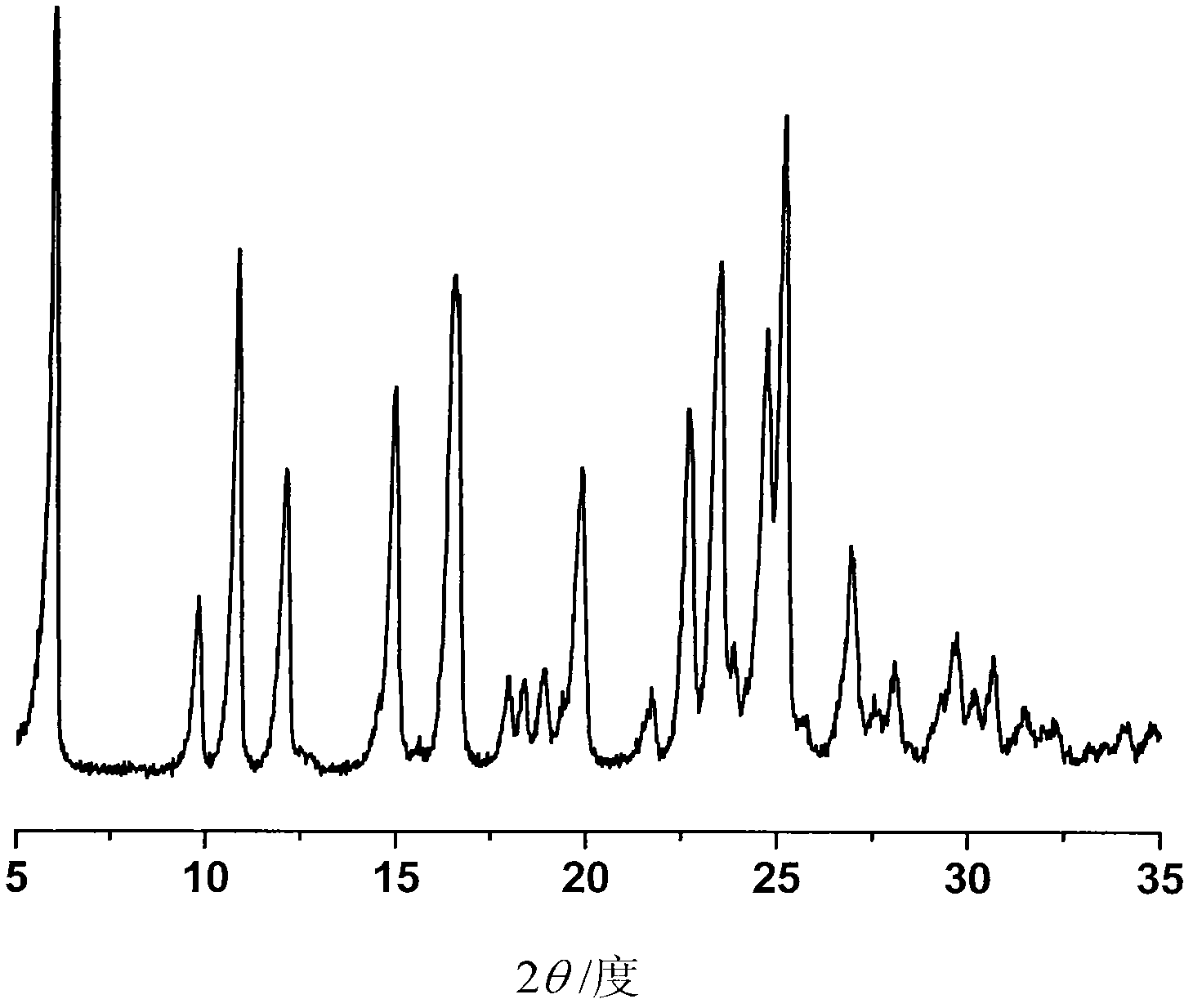
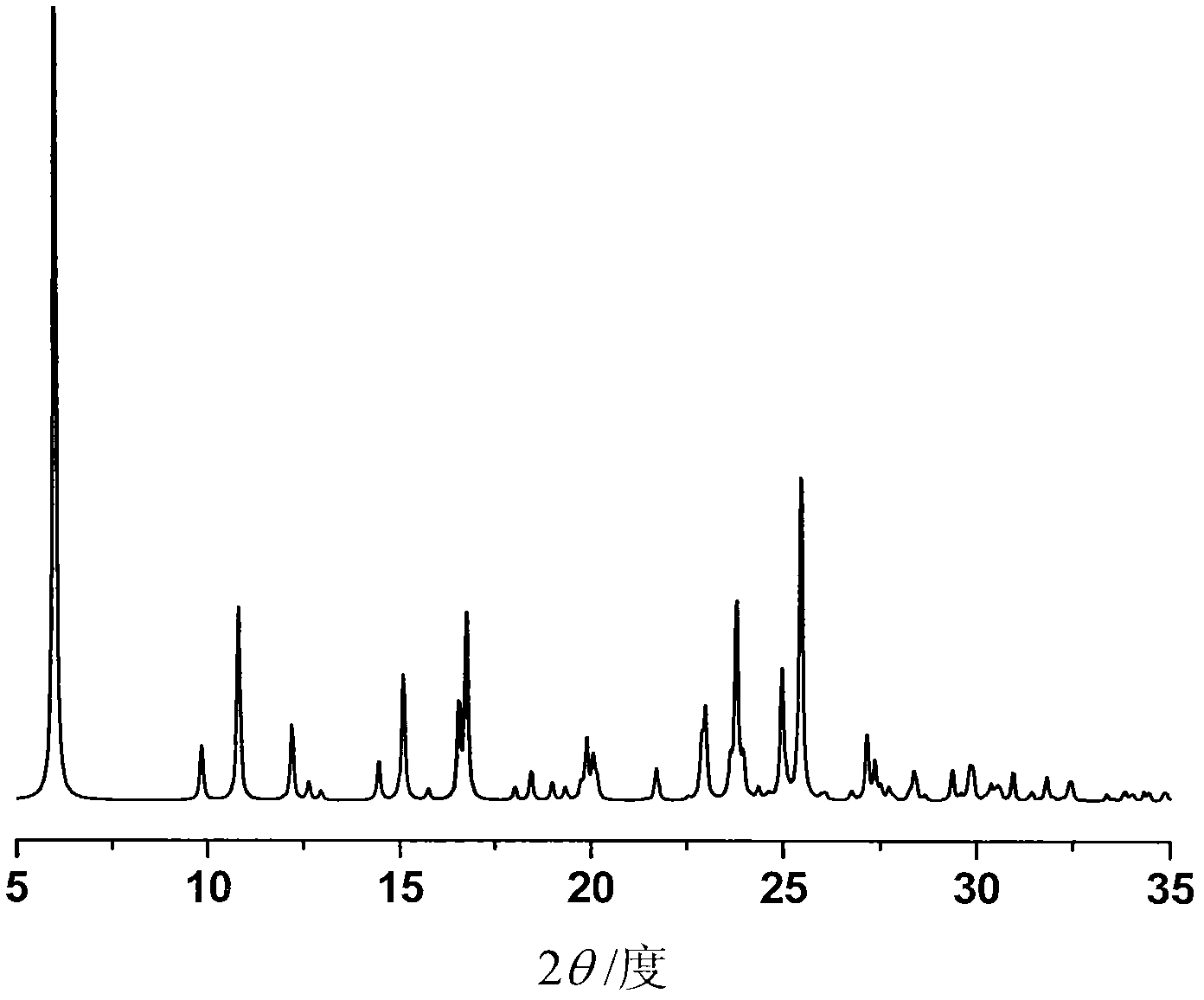
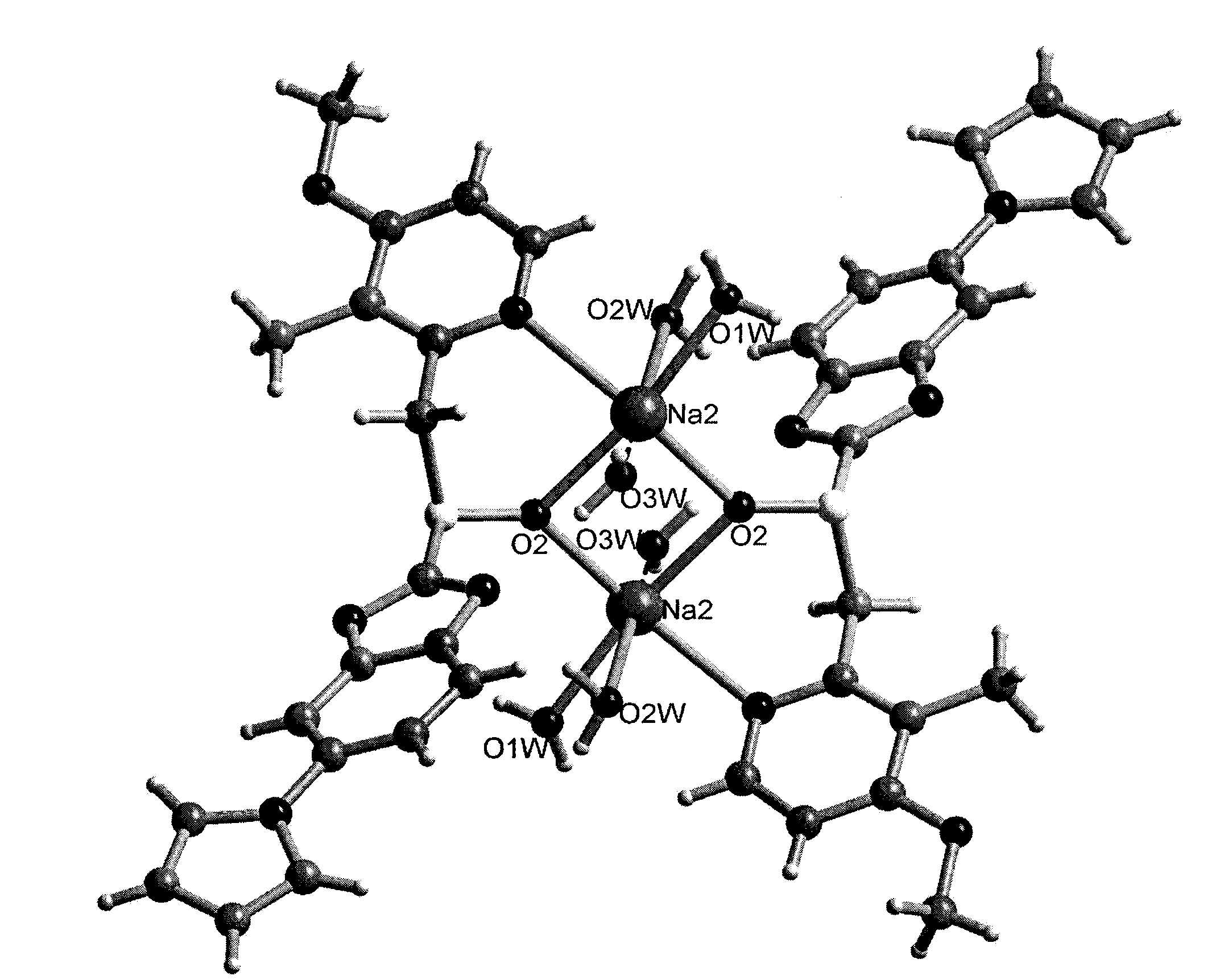
[0036] First put 33% aqueous sodium hydroxide solution on the lower layer of the test tube, dissolve 100 mg of ilaprazole in isopropanol to prepare a saturated solution, and then carefully add the solution dropwise to the upper layer of the test tube (v / v 1:1) , standing at room temperature for liquid-liquid diffusion for about a week, to obtain 80 mg of pale yellow needle-like crystals, with a yield of 80%.
Embodiment 2
[0038] Dissolve 100 mg of ilaprazole sodium in alkaline isopropanol at 40°C and pH 11 to make a saturated solution, filter, and slowly cool down to 4°C. After 12 hours, 86 mg of light yellow needle-shaped crystals were obtained, with a yield of 86%.
Embodiment 3
[0040] At room temperature, 100 mg of ilaprazole sodium was dissolved in isopropanol to make a saturated solution. At room temperature, the solution was placed in an isopropyl ether atmosphere for gas-liquid diffusion. After 24 hours, 85 mg of light yellow needle-shaped crystals were obtained, producing rate of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com