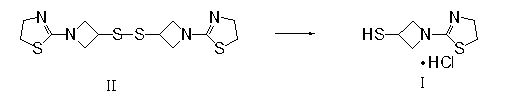
3-sulfhydryl-1-(1,3-thiazoline-2-yl)-azetidine hydrochloride crystal and preparation method thereof
An azetidine and thiazoline technology, applied in the field of organic drug synthesis, can solve the problems of easy moisture absorption, unstable chemical properties, unfavorable preservation and the like, and achieve the effects of less impurities, good quality and favorable preservation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
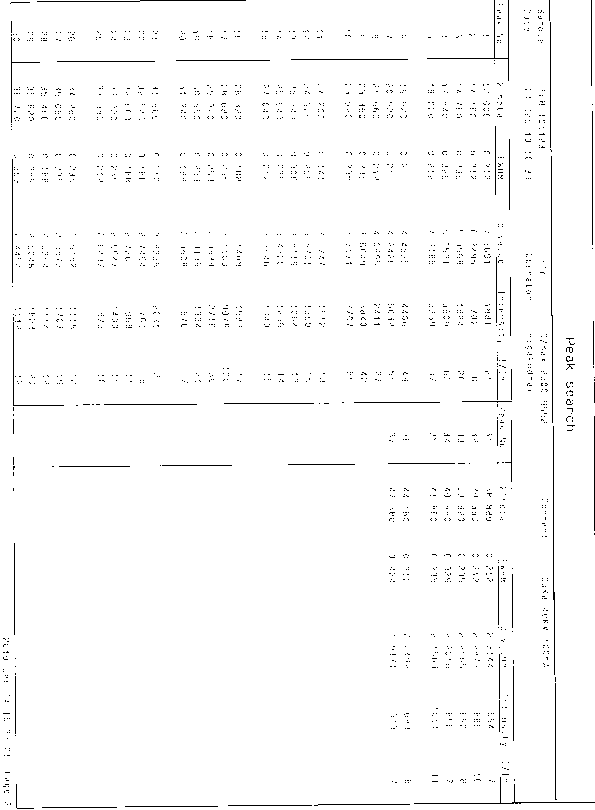
[0041] Add 20.8g (60mmol) bis-[1-(1,3-thiazoline-2-yl) azetidin-3-yl] disulfide (compound Ⅱ) in a 500ml three-necked flask, 36.4g ( 180mmol) tributylphosphine, 6.5ml (360mmol) water, 42ml (500mmol) concentrated hydrochloric acid, 200ml Virahol, stirred at 50°C for 8 hours, evaporated the solvent to dryness, obtained about 48g oil, added 455ml acetonitrile / isopropyl ether ( Volume ratio 1 / 12), stirred for 1 hour at 20°C, filtered to obtain 23.8g of white solid, see X-ray powder diffraction pattern figure 1 , yield 94%, melting point: 119-121.5°C.
Embodiment 2
[0043]In a 250ml three-necked flask, add 8.7g (25mmol) compound II, 17.7g (87.5mmol) tributylphosphine, 3.4ml (187.5mmol) water, 19ml (227.5mmol) concentrated hydrochloric acid, 120ml isopropanol, and stir at 55°C After 7 hours, the solvent was evaporated to dryness to obtain about 18g of oily substance, and 160ml of methanol / dichloromethane (volume ratio 1 / 10) was added, stirred at 10°C for 1 hour, filtered, and 10.1g of white solid was obtained by filtration, X-ray powder diffraction pattern See figure 1 , yield 95%, melting point: 119.5-121.5°C.
Embodiment 3
[0045] In a 250ml three-necked flask, add 5.2g (15mmol) compound II, 12.5g (62mmol) tributylphosphine, 2.6ml (144mmol) water, 13ml (155mmol) concentrated hydrochloric acid, 95ml isopropanol, and stir at 45°C for 7 hours. Evaporate the solvent to dryness to obtain about 10g of oil, add 75ml of methanol / methyl isobutyl ketone (volume ratio 1 / 3), stir at 10°C for 1 hour, filter to obtain 5.8g of white solid, X-ray powder diffraction pattern See figure 1 , yield 93%, melting point: 119-121°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com