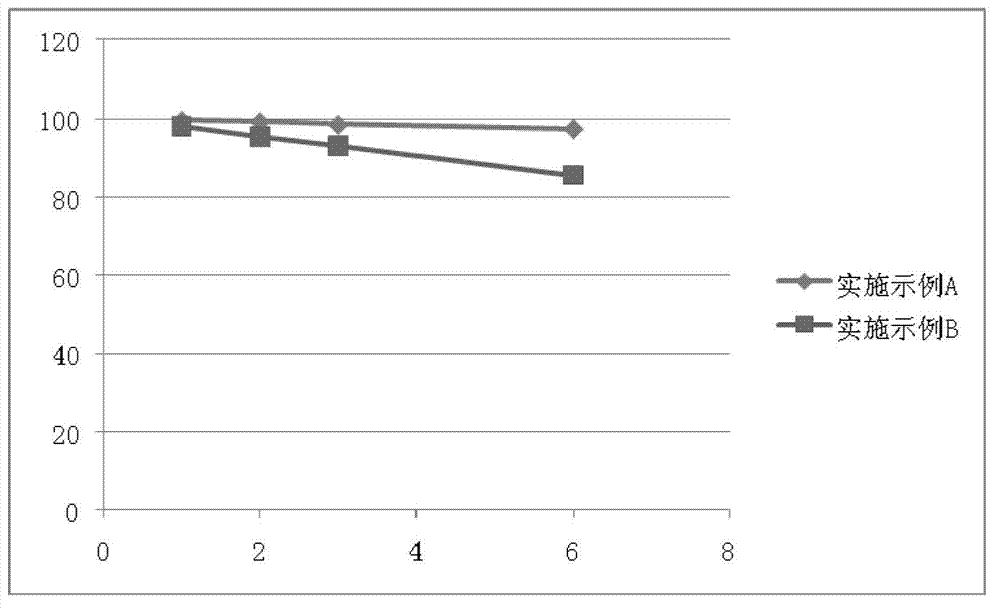
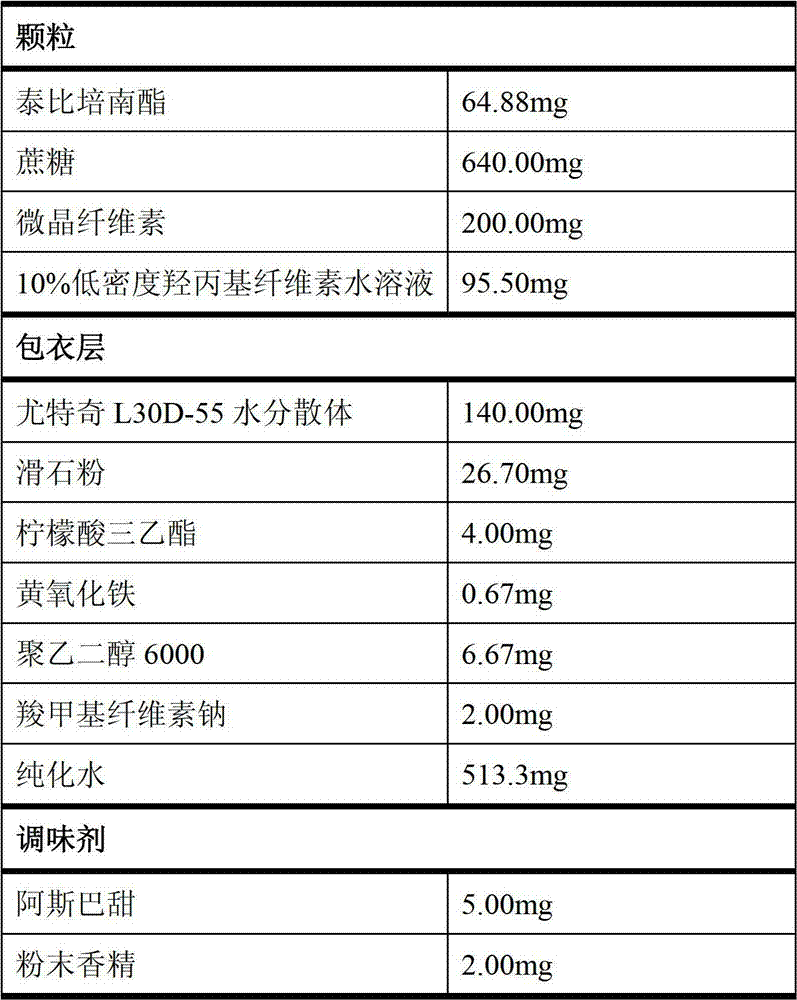
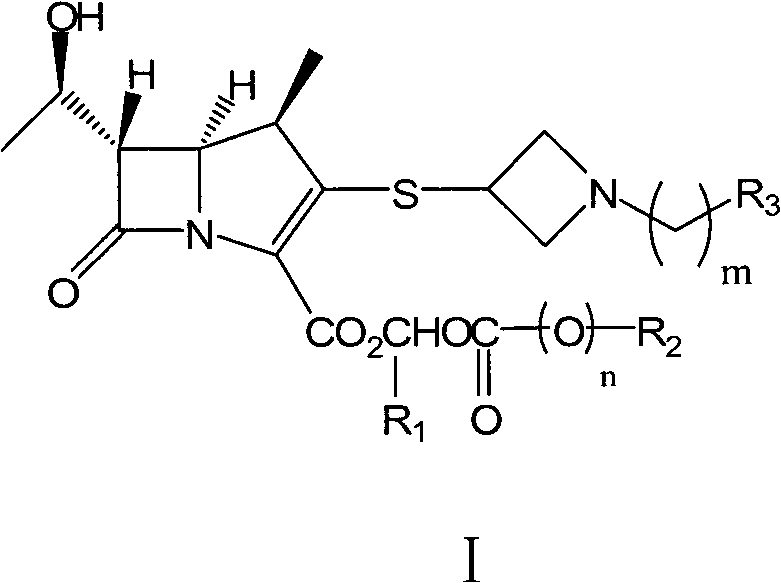
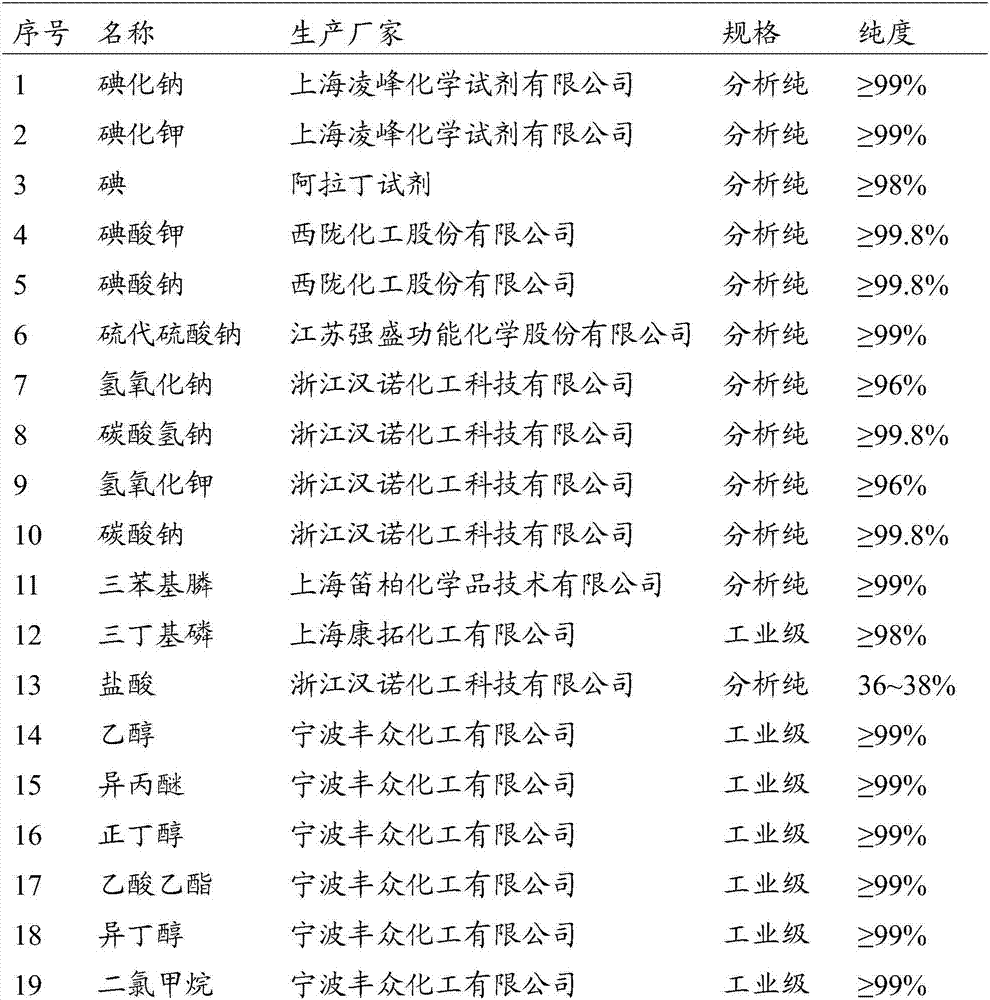
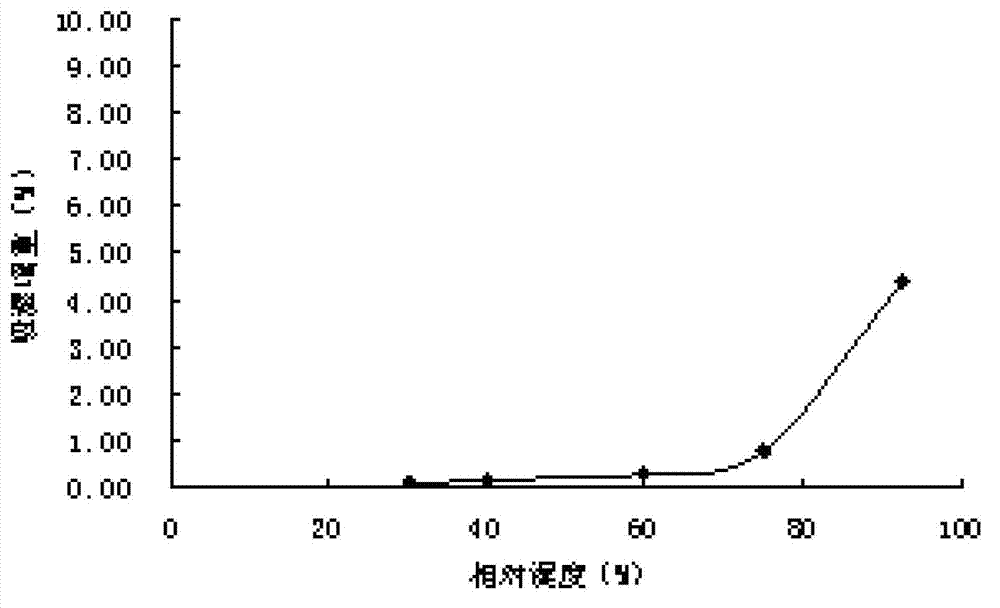
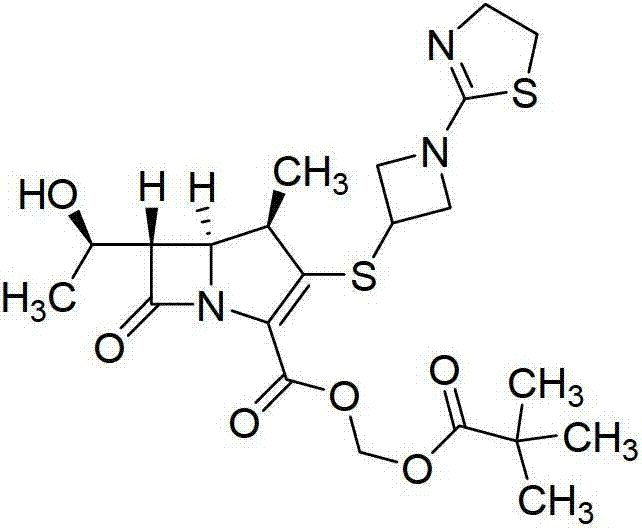
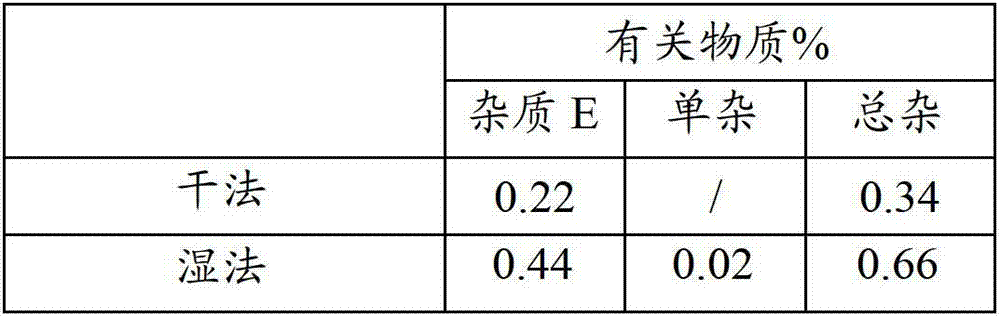
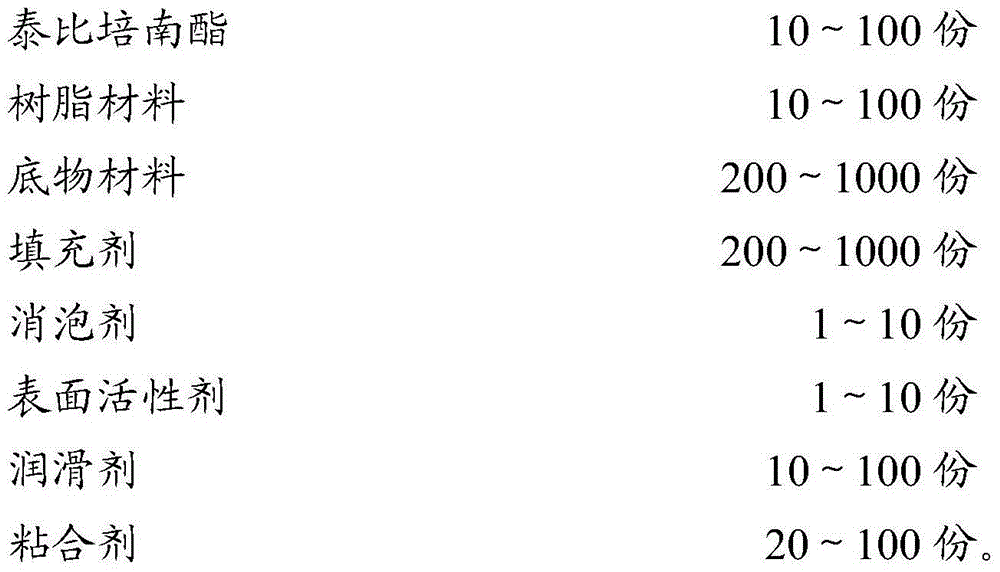Patents
Literature
61 results about "TEBIPENEM PIVOXIL" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
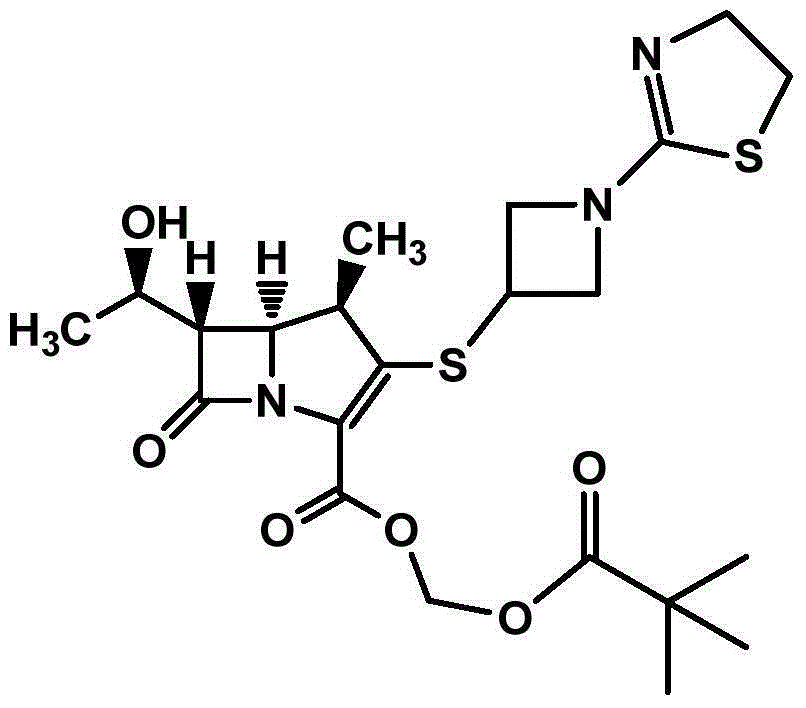
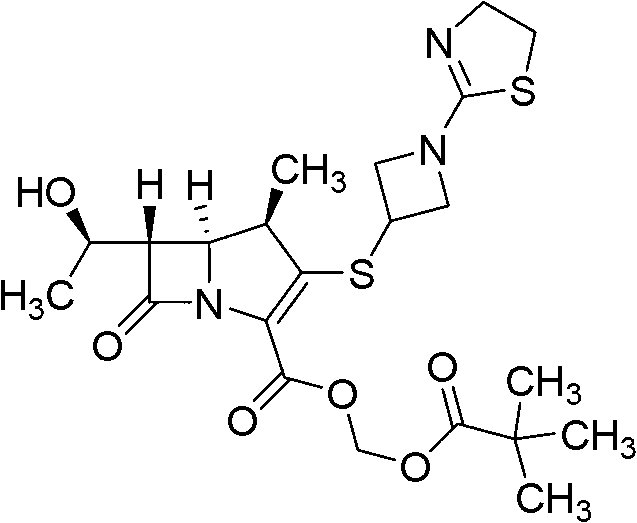
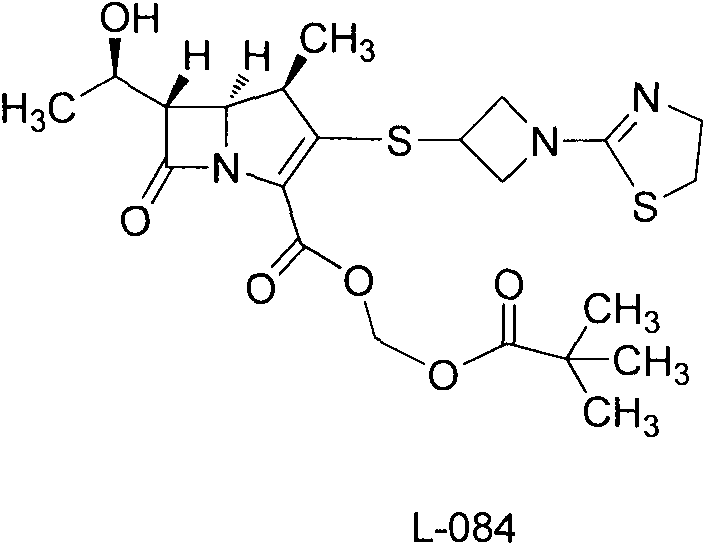
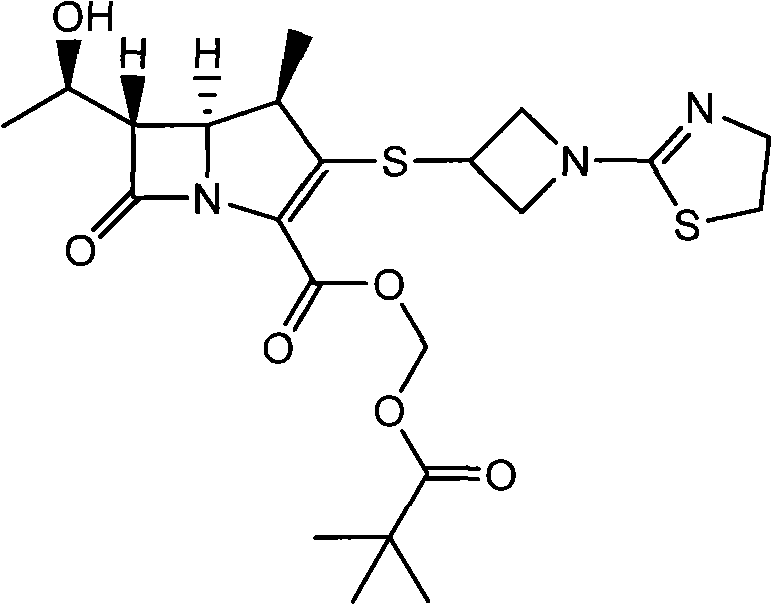
Tebipenem (brand name Orapenem) is a broad-spectrum orally-administered antibiotic, from the carbapenem subgroup of β-lactam antibiotics. ... Tebipenem is formulated as the ester tebipenem pivoxil due to the better absorption and improved bioavailability of this form.
Tebipenem pivoxil oral solid preparation and preparation method thereof
ActiveCN103054815AAffect quality issuesEnsure safetyAntibacterial agentsOrganic active ingredientsDrug productTEBIPENEM PIVOXIL
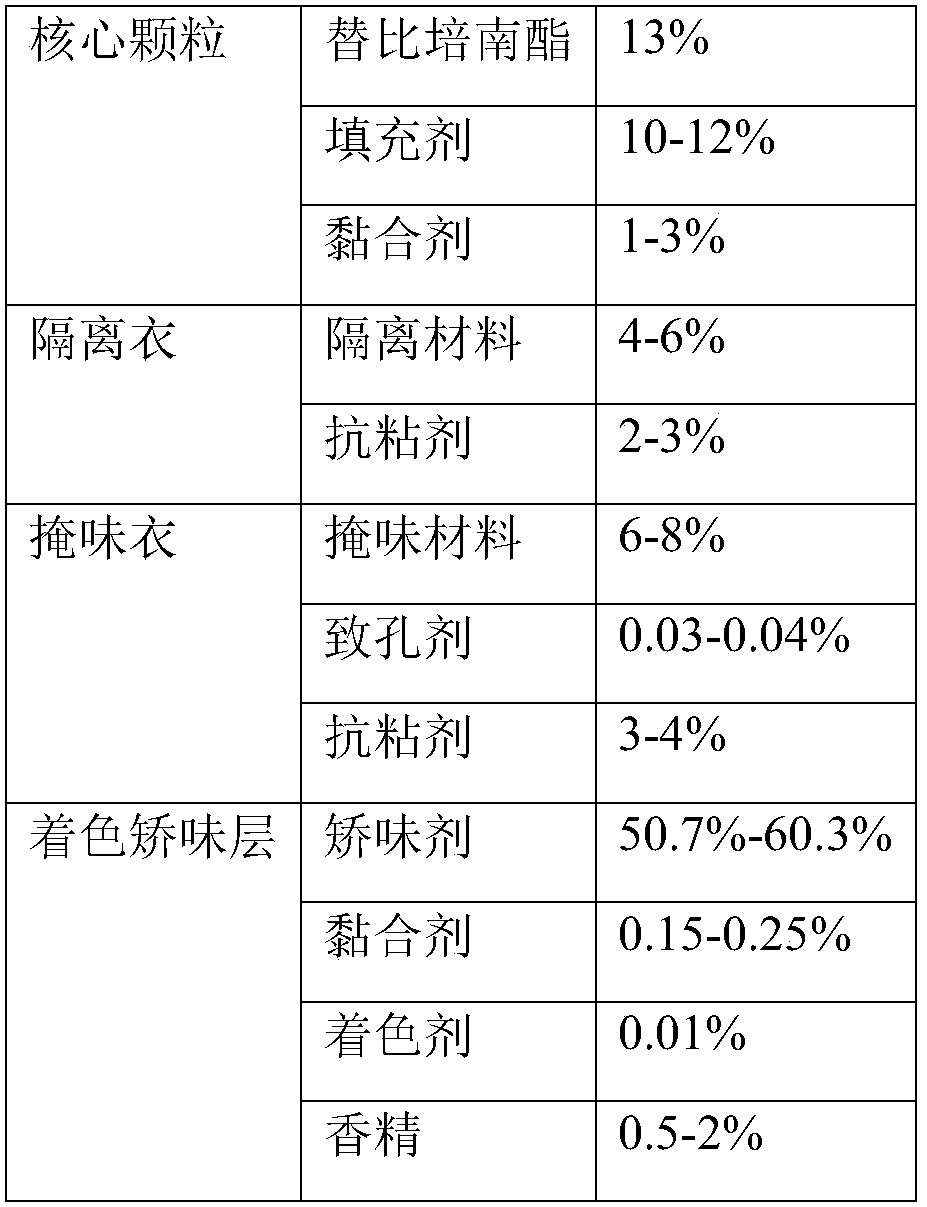
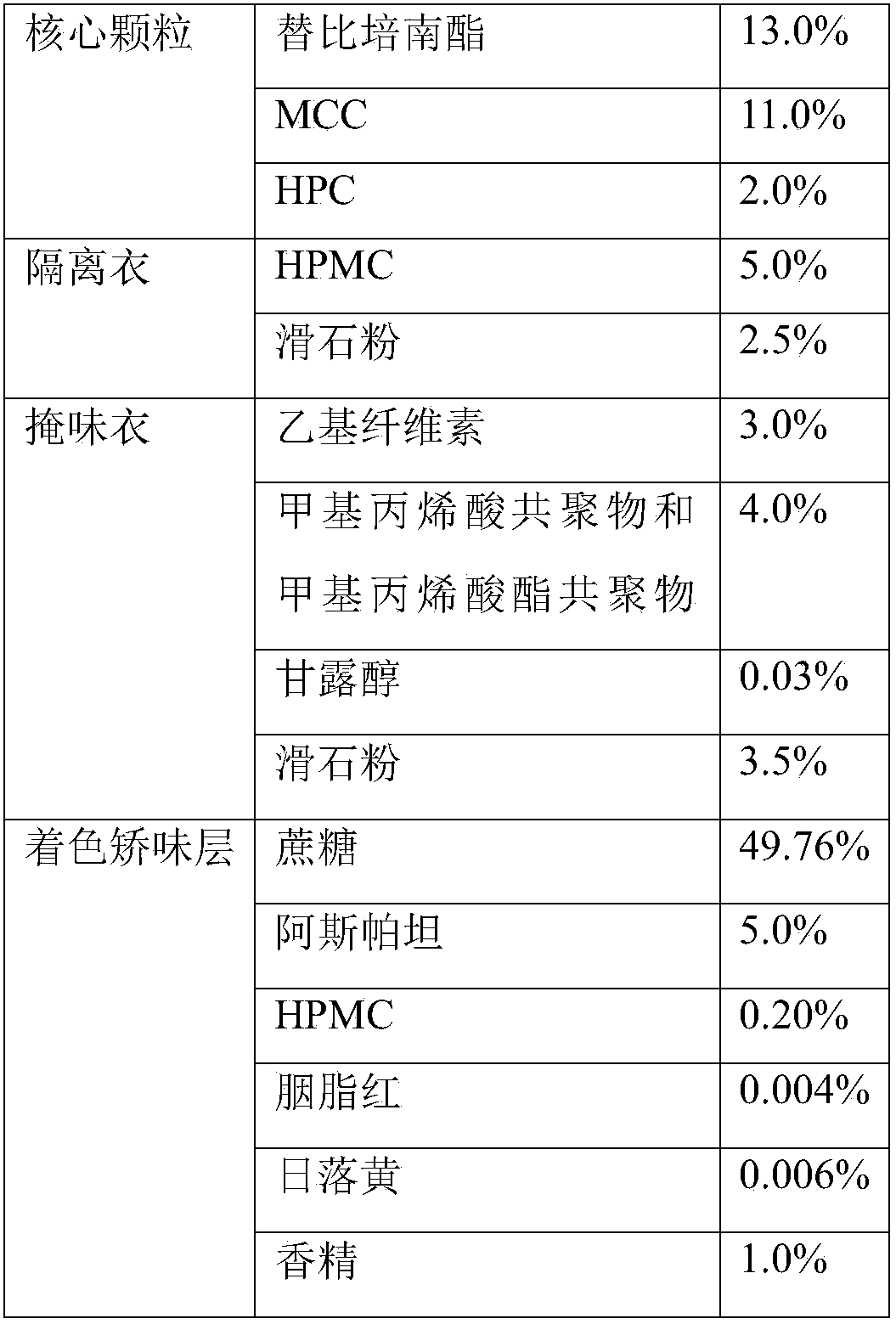
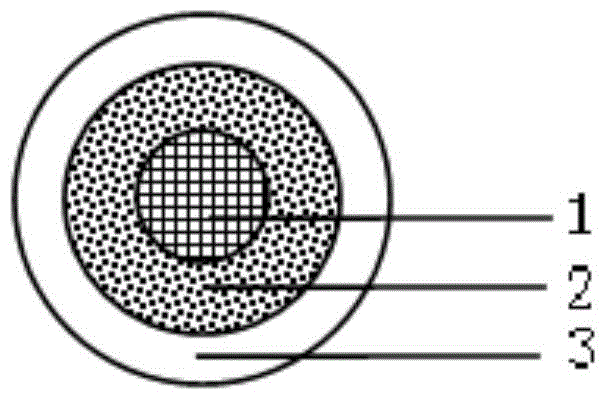
The invention discloses a tebipenem pivoxil oral solid preparation and a preparation method thereof. The oral solid preparation disclosed by the invention comprises particles, an isolation coating layer, a taste masking coating layer and a coloring tender taste coating layer, wherein the particles are prepared from tebipenem pivoxil, microcrystalline cellulose and binders. According to the invention, the preparation can be used for overcoming the problem that a part of water in main medicines and excipients has an interaction to affect the quality of a finished product, and is suitably prepared into a medicine.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Tebipenem pivoxil granule composition as well as preparation method and application thereof
InactiveCN105193742APreparation Technology ScienceReasonable preparation processAntibacterial agentsOrganic active ingredientsInsulation layerSucrose
The invention provides stable tebipenem pivoxil granule composition. The composition comprises tebipenem pivoxil raw material and pharmaceutical excipients, wherein the pharmaceutical excipients comprise a filler, an adhesive and a coating solution; the coating solution comprises an insulation layer, a taste mask layer and a coloring and taste modifying layer; the filler comprises sucrose and microcrystalline cellulose, the amount of the sucrose accounts for 50%-70% of the total weight of the granule composition, and the amount of the microcrystalline cellulose accounts for 5%-15% of the total weight of the granule composition. The invention further provides a preparation method of the composition and an application of the composition in preparation of drugs with an antibacterial function.
Owner:HAIKOU PHARMA FACTORY
Tebipenem pivoxil oral preparation and preparation method thereof
ActiveCN102860985AGreat tasteImprove medication complianceAntibacterial agentsPowder deliveryActive componentPatient compliance
The invention relates to a tebipenem pivoxil-containing oral preparation and a preparation method of the tebipenem pivoxil-containing oral preparation. The preparation provided by the invention consists of tebipenem pivoxil and a pharmaceutic adjuvant as the active components. The pharmaceutic adjuvant contains beta-cyclodextrin. The tebipenem pivoxil oral preparation provided by the invention overcomes the defects that the grit feeling is produced when the tebipenem pivoxil oral preparation is taken, the patient compliance is poor and the preparation technology is complicate in the prior art, so that the tebipenem pivoxil oral preparation is an excellent preparation with good taste, high dissolvability, good stability and simple preparation technology.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Oral preparation containing tebipenem pivoxil
ActiveCN102885811ASuitable for childrenImprove stabilityAntibacterial agentsOrganic active ingredientsAdhesiveBitter taste
An oral preparation containing tebipenem pivoxil is characterized by comprising the following components in percentage by weight: 1 to 20 percent of tebipenem pivoxil, 60 to 98 percent of filler, 0.5 to 10 percent of adhesive and 3 to 50 percent of coating agent; and only water is taken as a solvent in the preparation of the oral preparation. The bitter taste of raw medicines is covered by using a taste-covering technology, and the oral preparation is suitable for children. In a whole preparation process, only water is taken as the solvent, so that stability and safety of the preparation in preparation and storage processes are guaranteed.
Owner:NANJING HUAWE MEDICINE TECH DEV
Preparation method of carbapenems
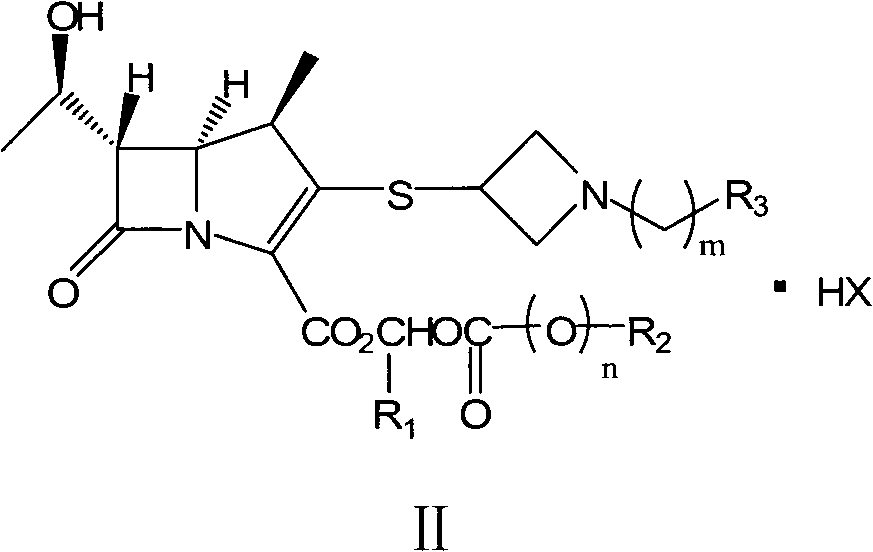
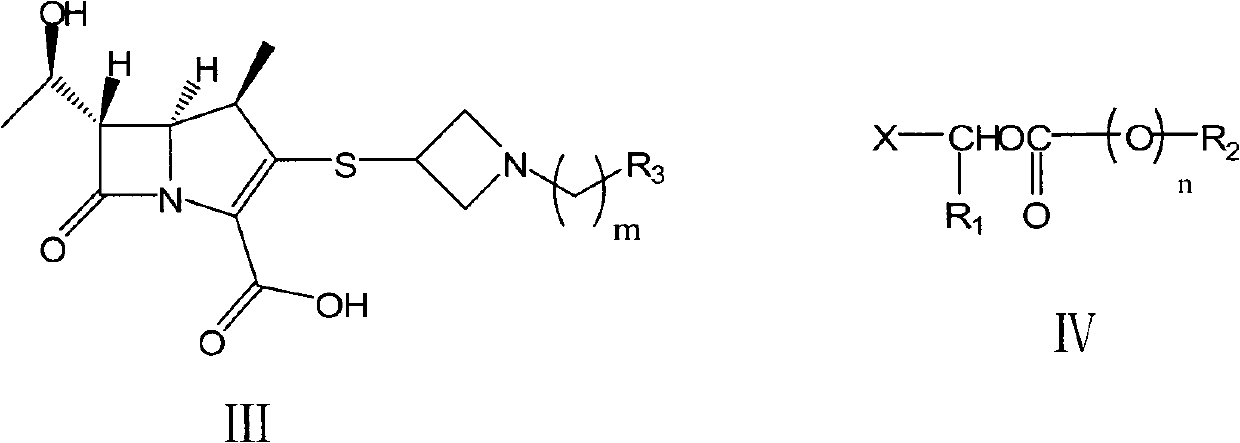
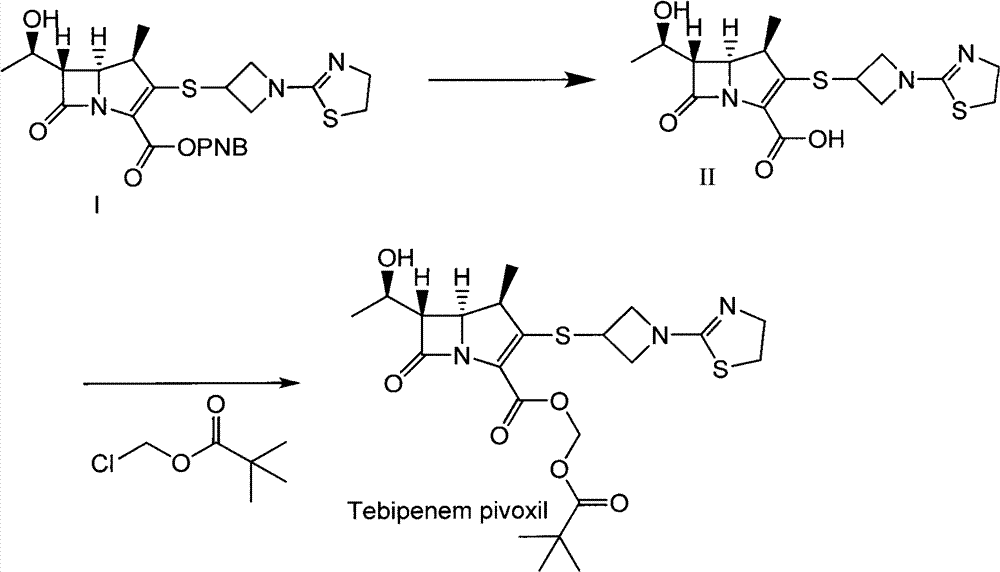
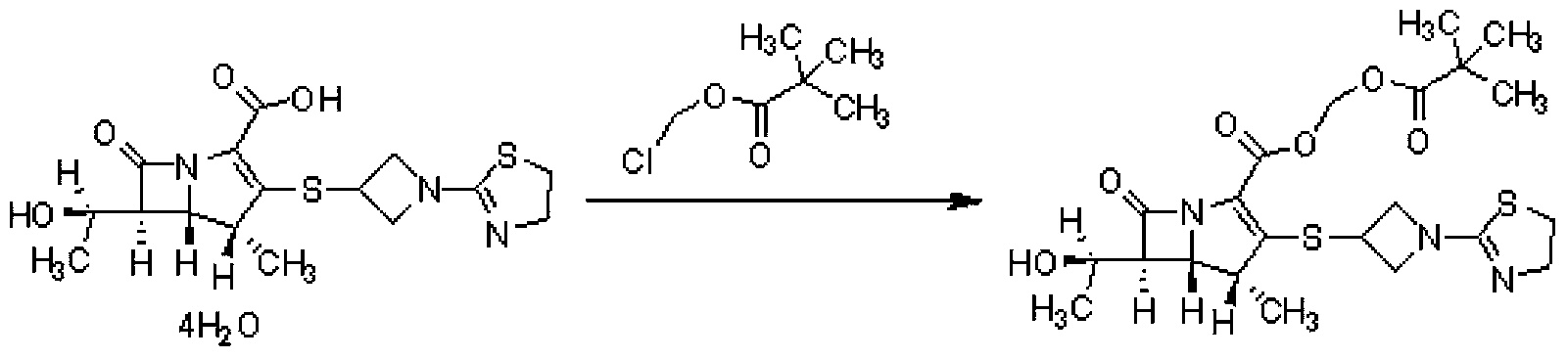
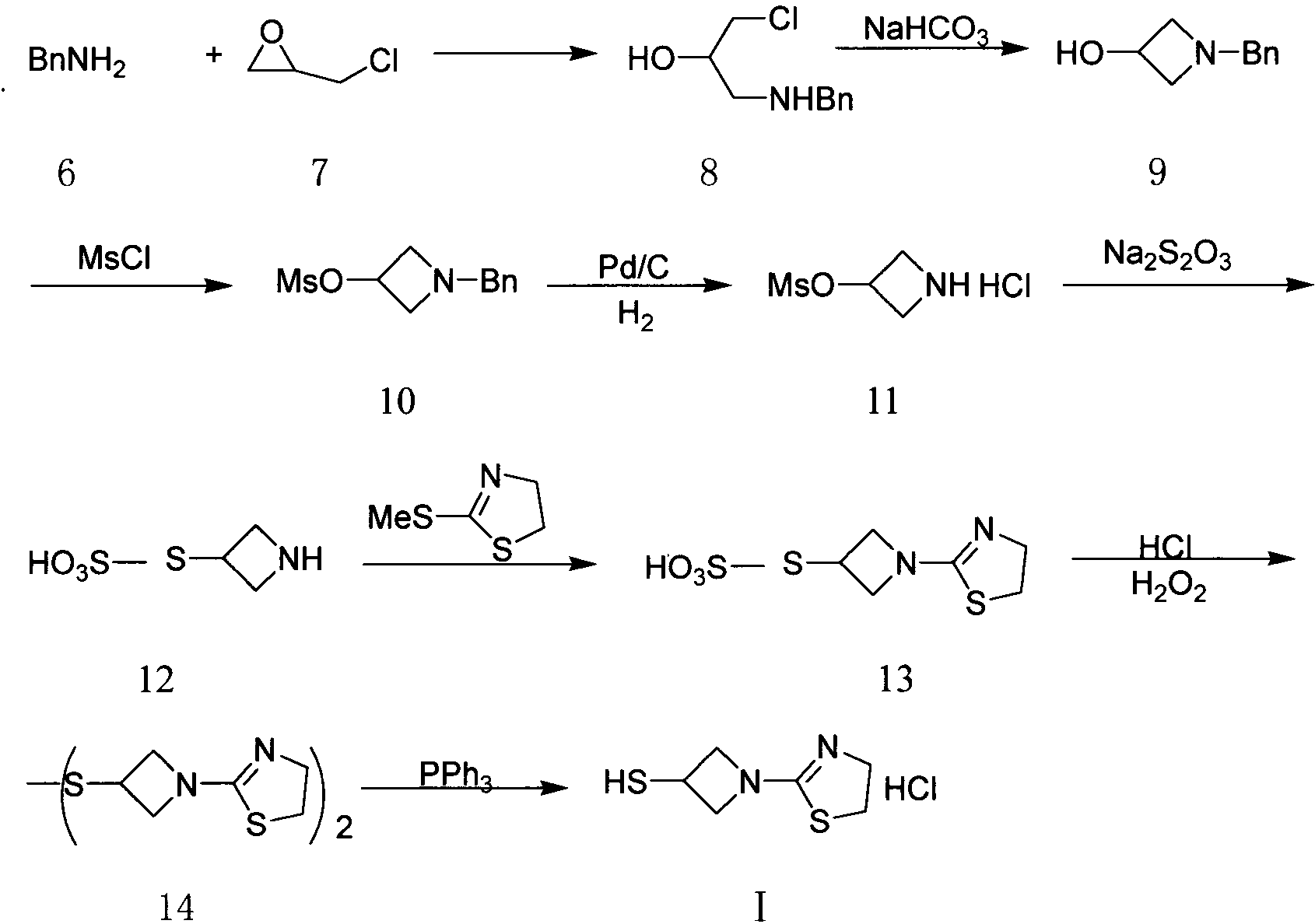
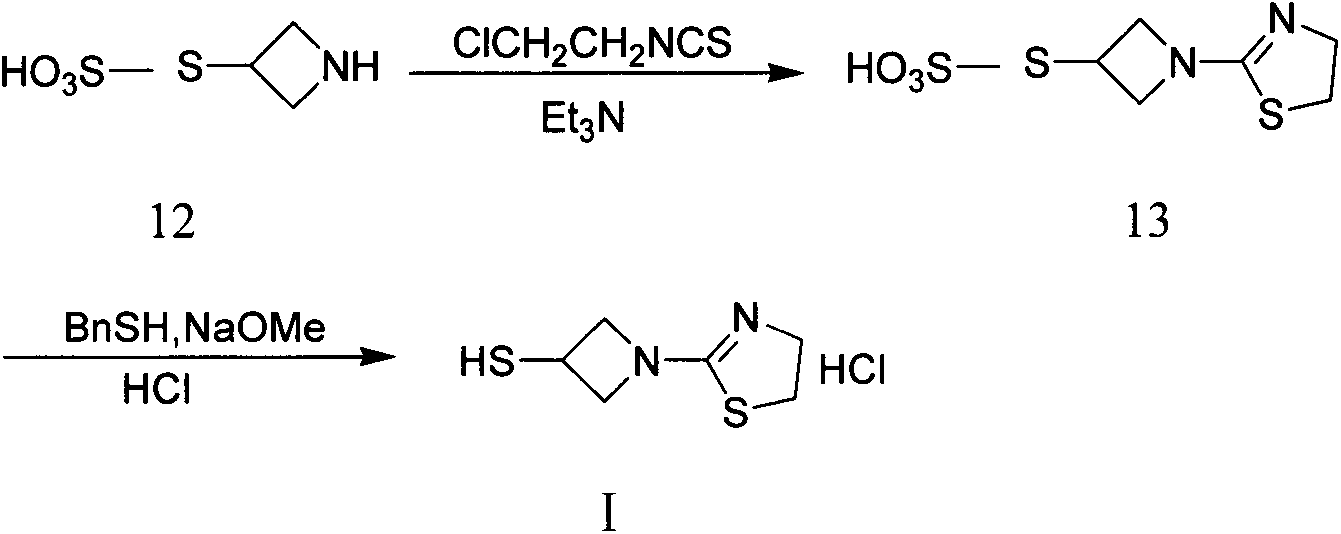
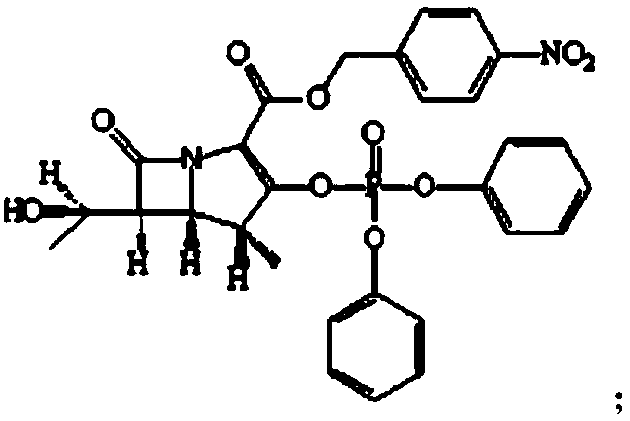
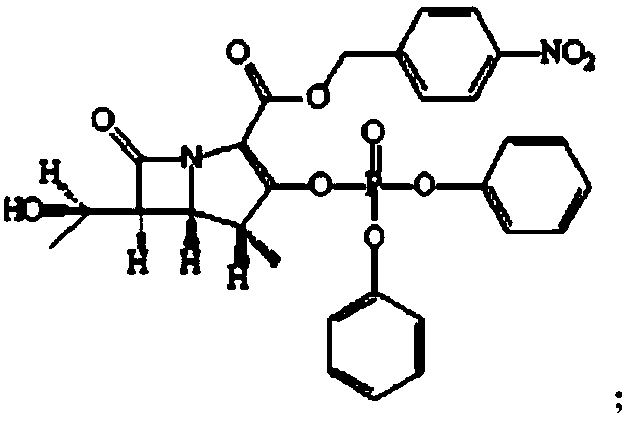
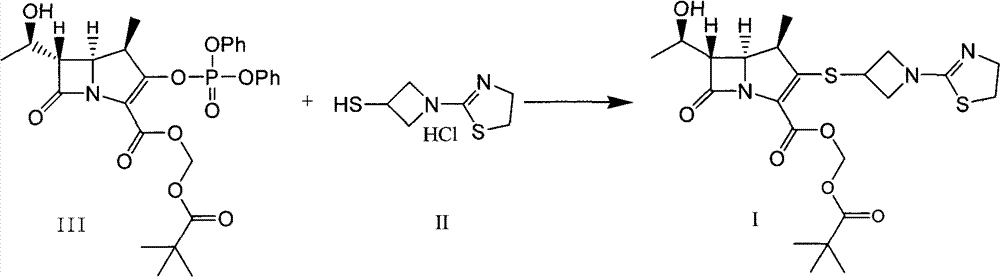
The invention relates to a preparation method of tebipenem pivoxil and analogue thereof. In the method, carbapenem compound salt with a formula II is used as a raw material to obtain carbapenem compound ester with a formula I under the action of alkali, wherein the carbapenem compound salt with the formula II is prepared by direct reaction of a carbapenem compound with a formula III and ester with a formula IV. According to the invention, high-yield and high-purity salt of tebipenem pivoxil and analogue thereof can be prepared, so that high-yield and high-purity tebipenem pivoxil or analogue thereof can be obtained without column chromatography. Thus, the method provided by the invention is more suitable for industrial production in a large scale.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Preparation method of tebipenem pivoxil
ActiveCN103059028AHigh yieldSuitable for industrial productionOrganic chemistryChemical synthesisPhosphate
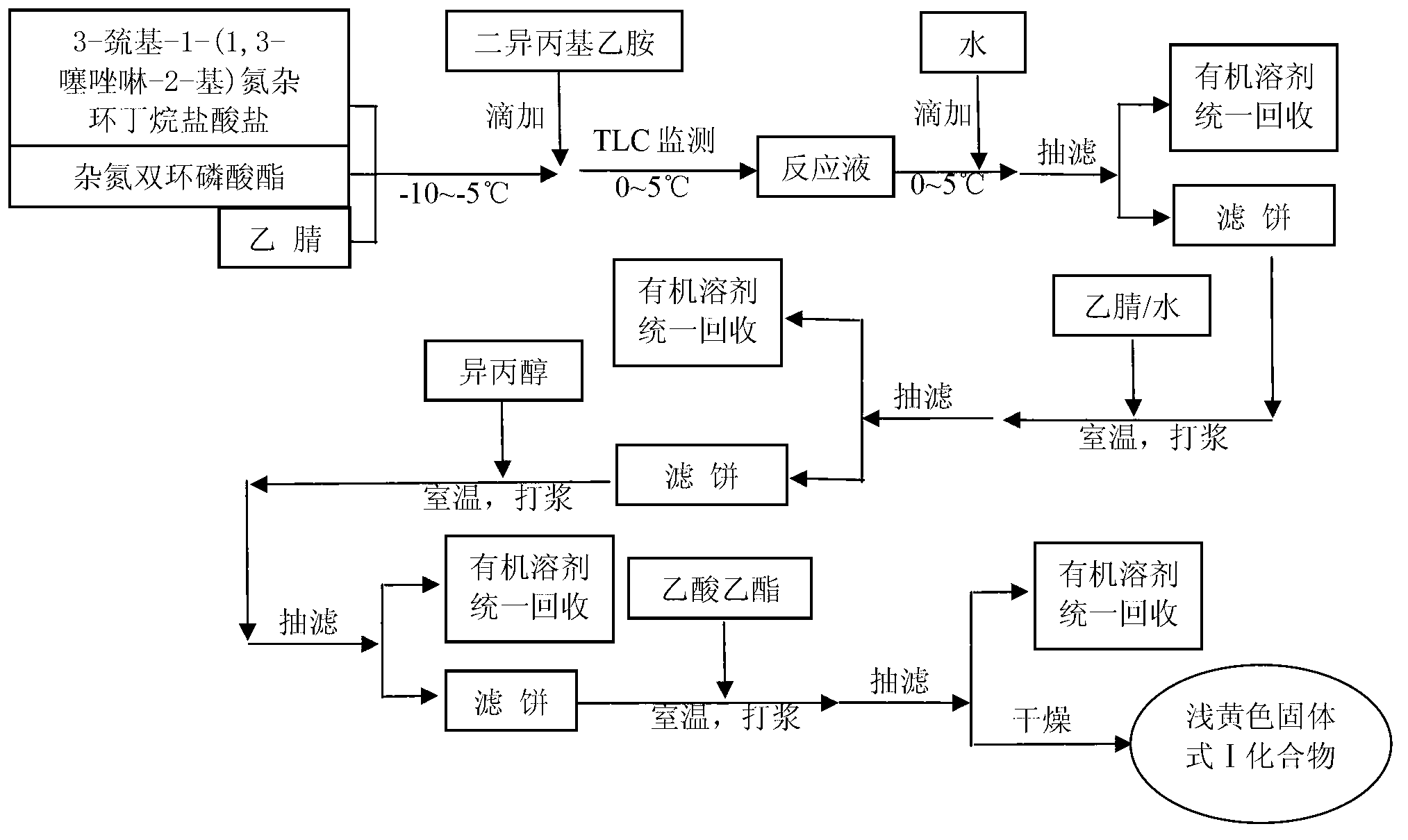
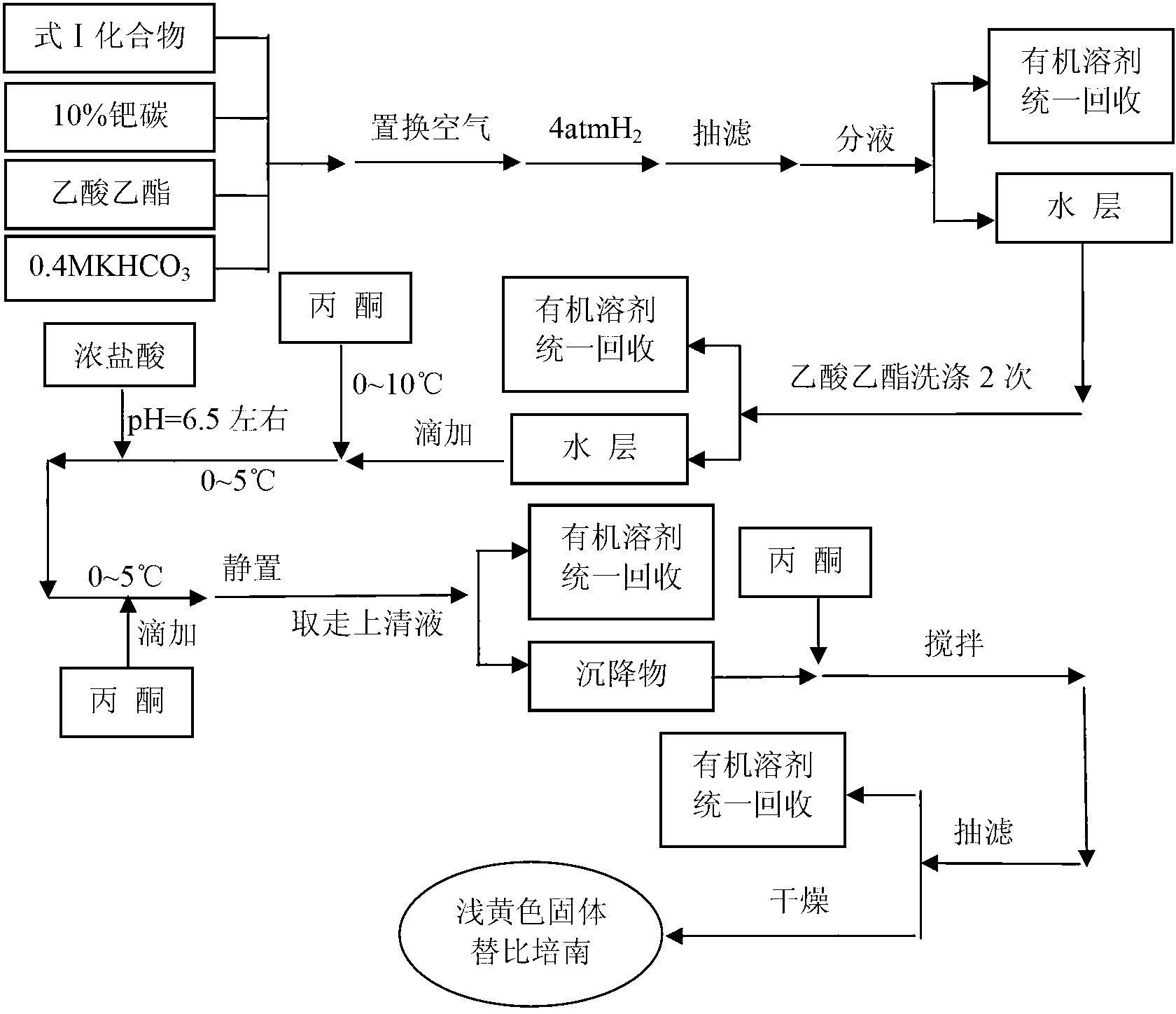
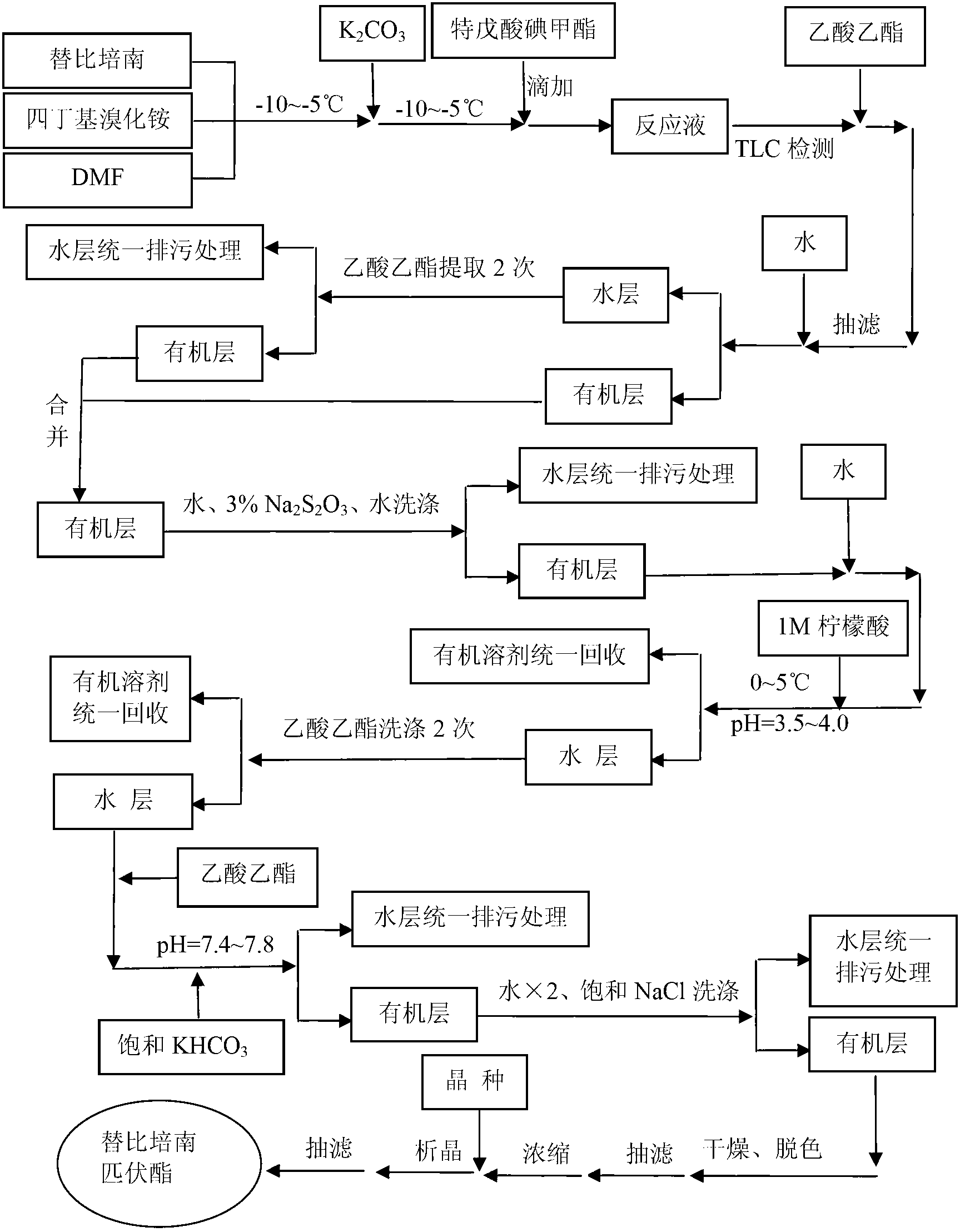
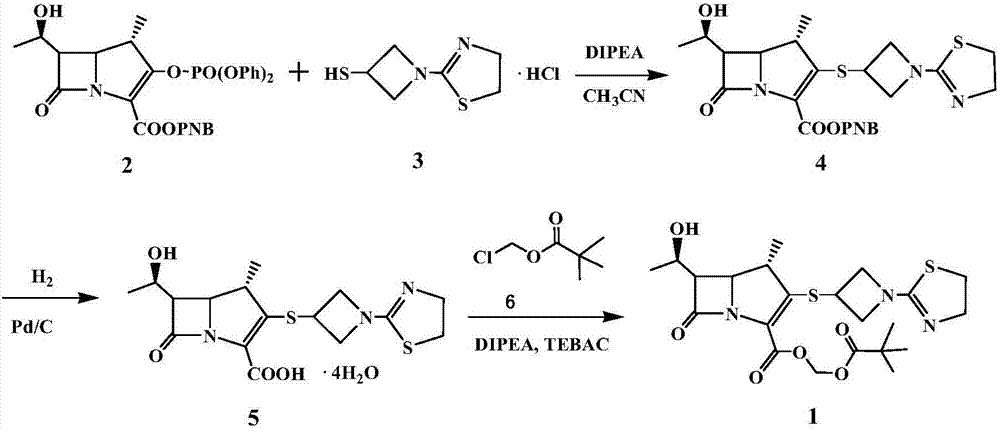
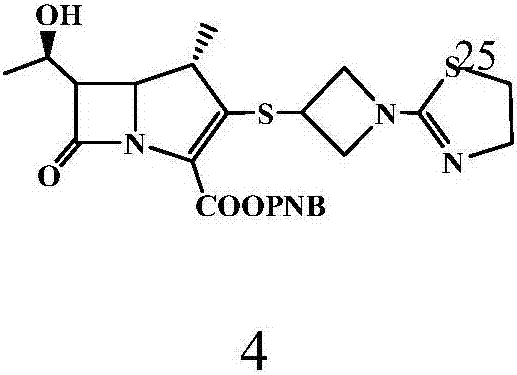
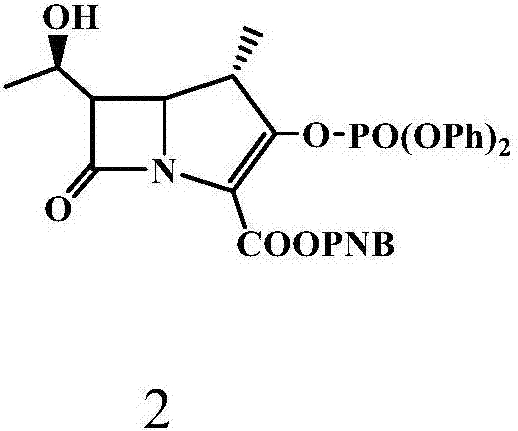
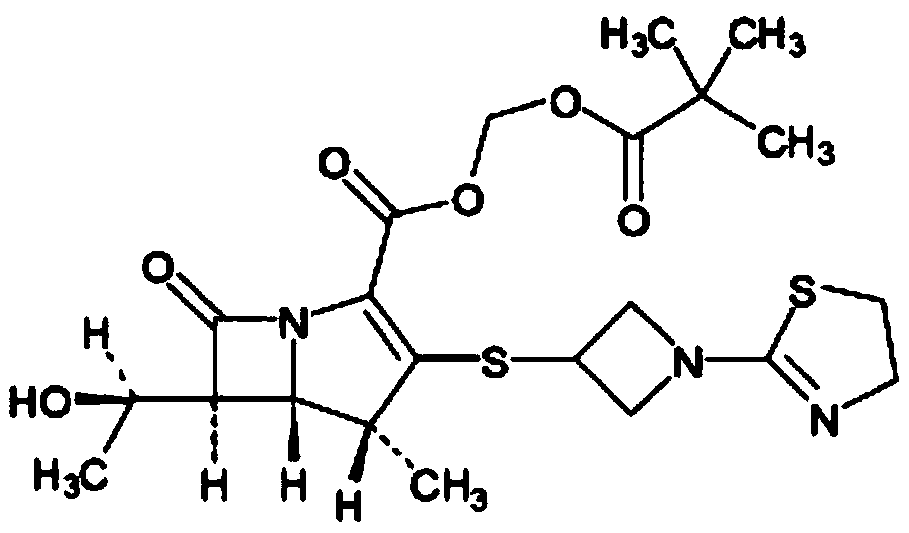
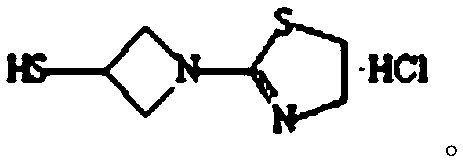
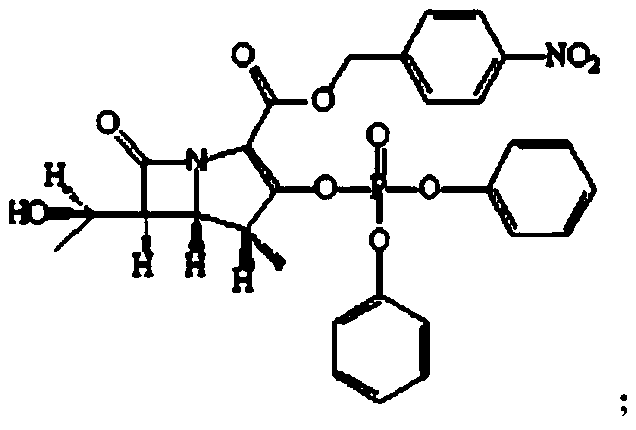
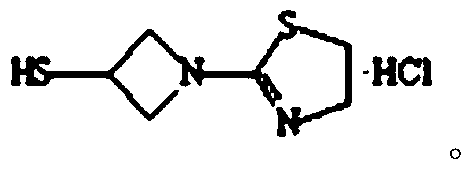
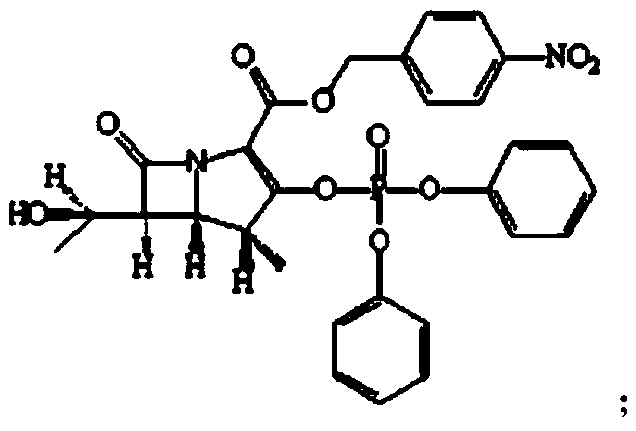
The invention relates to the field of chemical synthesis, and discloses a preparation method of tebipenem pivoxil. The preparation method comprises the following steps of: performing condensation reaction on azabicyclo phosphate and 3-mercapto-1-(1,3-thiazoline-2-yl) azetidine hydrochloride by taking acetonitrile as a solvent in the presence of diisopropylethylamine; in a mixed solvent consisting of acetic ether and a potassium bicarbonate aqueous solution, performing hydrogenization on the compound shown by formula I to remove p-nitrobenzyl to obtain tebipenem; and under the catalysis of a phase transfer catalyst, adding anhydrous potassium carbonate into the tebipenem and iodomethyl pivalate to perform condensation reaction to obtain the tebipenem pivoxil. By adopting the preparation method, according to the defects of related solvents and operations of purification and the like in the conventional method for preparing the tebipenem pivoxil, co-adapted solvents and purification operations are selected during preparation according to a reaction mechanism, so that the yield of the tebipenem pivoxil is improved, and the preparation method is suitable for industrial production. The formula I is shown in the description.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
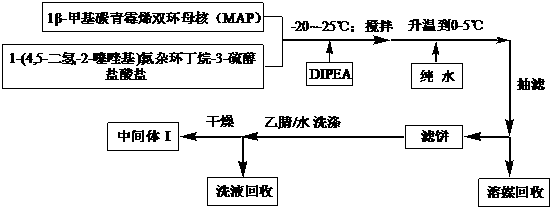
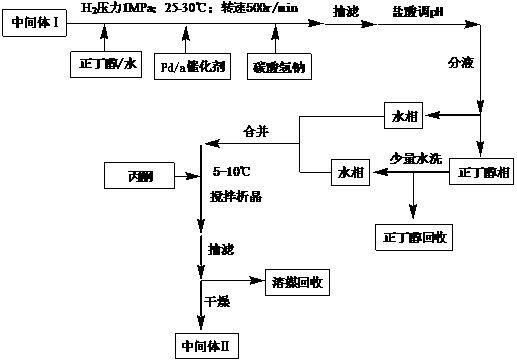
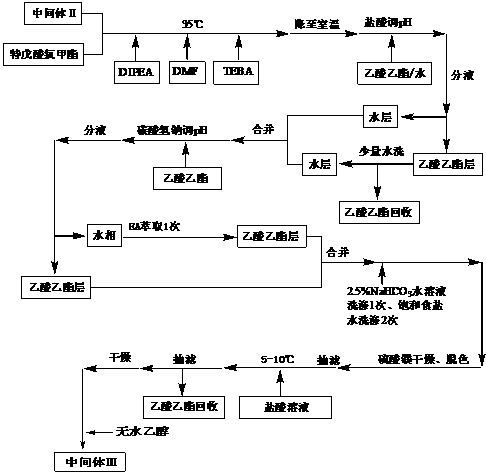
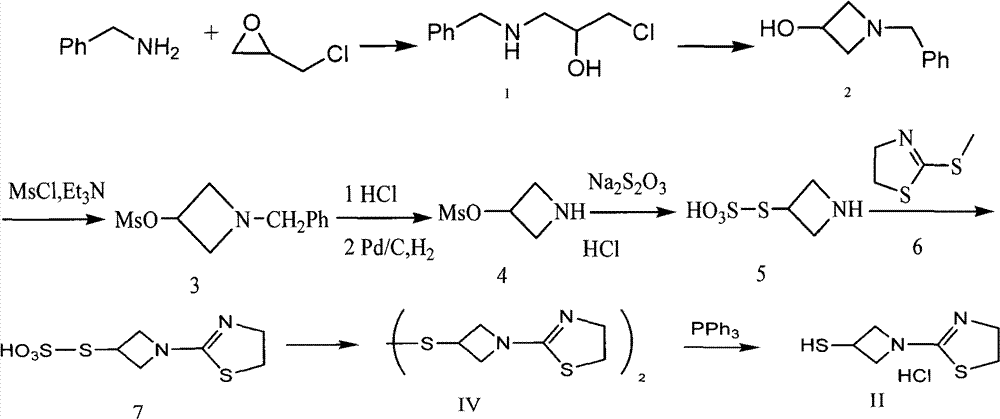
Preparation method for intermediate of tebipenem pivoxil
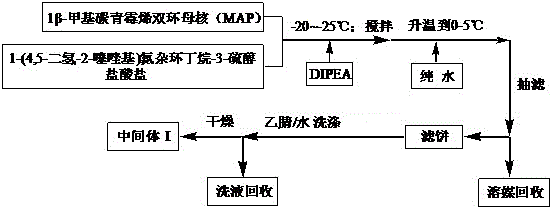
The invention discloses a preparation method for an intermediate of tebipenem pivoxil shown as formula 1. The preparation method comprises the following step of performing the following reaction on a compound MAP and a compound IV in an organic solvent and water, with the action of (R1)3P and an organic base, under inert gas protection, wherein the volume ratio of the organic solvent and water is 1000 : 1-10 : 1; a reaction temperature is -40-50 DEG C; and R1 represents phenyl or C2-C8 alkyls. The preparation method is easy to operate, simple in post-treatment, with the product having high yield and high purity, and is suitable for industrialized production.
Owner:SHANGHAI INST OF PHARMA IND
Preparation method of tebipenem pivoxil and intermediate thereof
ActiveCN107501268AHigh purityMild reaction conditionsOrganic chemistryReaction temperatureTEBIPENEM PIVOXIL
The invention provides a preparation method of tebipenem pivoxil. A three-step method synthesis route taking MAP (compound 2) and TAT (compound 3) as starting raw materials is adopted. The preparation method is characterized in that in the first step, the temperature of reaction for preparing an intermediate compound 4 of the tebipenem pivoxil from the MPA and the TAT is -4 to 5 DEG C. Compared with the preparation method using the three-step method synthesis route in the prior art, the preparation method has the following advantages: the step avoids ultralow reaction temperature, so that the method is mild in reaction condition, easy to implement and suitable for industrialized production; furthermore, the reaction yield in each step is increased and the purify of reactants is improved.
Owner:ZHEJIANG HISOAR CHUANNAN PHARMA +1
Preparation method of fine tebipenem pivoxil granules
ActiveCN109432044AImprove stabilityImprove the poor taste of medicines and other problemsAntibacterial agentsOrganic active ingredientsFlavorPrill
The invention relates to a preparation method of fine tebipenem pivoxil granules. Each fine tebipenem pivoxil granule comprises four parts as follows: a core granule, an isolation coat, a taste masking coat and a coloring and corrigent layer, wherein the core granule is prepared with a high shear mixed granulator, the isolation coat and the taste masking coat are prepared with a fluidized bed cutting and spraying technology, after the coloring and corrigent layer, a taste masking granule, a corrigent, a colorant and flavor are added, the granule is prepared with the high shear mixed granulator; the obtained granules are uniform in size, drug dissolution can be guaranteed, drug stability is improved, further, bad smell of the drug can be masked, and the granules are suitable for children; the adopted preparation process is simple, continuous proceeding of the process can be guaranteed, and the method can be applied to mass production.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Pharmaceutical preparation of tebipenem pivoxil composition and preparation method of pharmaceutical preparation
ActiveCN104013583APoor maskingHigh dissolution rateAntibacterial agentsOrganic active ingredientsFiller ExcipientDissolution
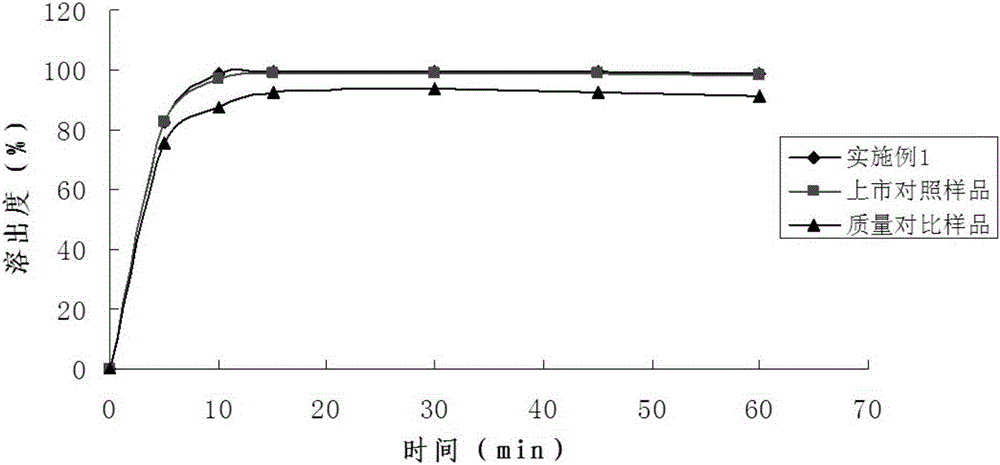
The invention belongs to the technical field of pharmaceutical preparations and discloses a pharmaceutical preparation of a tebipenem pivoxil composition and a preparation method of the pharmaceutical preparation. The pharmaceutical preparation of the tebipenem pivoxil composition comprises the following components in parts by weight: 10-100 parts of tebipenem pivoxil, 10-100 parts of a resin material, 200-1000 parts of a substrate material, 200-1000 parts of a filler, 1-10 parts of a defoamer, 1-10 parts of a surfactant, 10-100 parts of a lubricant and 20-100 parts of a binder. The pharmaceutical preparation of the tebipenem pivoxil composition has good dissolution rate and stability and also covers the bad taste of tebipenem pivoxil, and has a good taste. The preparation method provided by the invention further improve the dissolution rate of medicines, and the bitterness caused by tebipenem pivoxil is effectively covered, the effectiveness and safety of the preparation are guaranteed and the medicament compliance of the patient is improved.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Tebipenem pivoxil granule and preparation method thereof
InactiveCN104224725AQuality improvementEasy to takeAntibacterial agentsOrganic active ingredientsCarboxymethyl starchFluidized bed drying
The invention relates to a tebipenem pivoxil granule and a preparation method thereof. The granule is prepared according to the following steps: uniformly mixing tebipenem pivoxil, microcrystalline cellulose, lactose and carboxymethyl starch sodium, adding water to prepare a soft material, putting in a ball rolling machine for extruding and preparing pills, and drying by using a fluidized bed, and dressing. The dressing layer comprises a resin layer and a powder coating. The preparation method is capable of guaranteeing medicine stability through a special dressing prescription under the prerequisite of keeping the dissolution effect, and also is capable of masking the bitterness of the granule and improving the mouthfeel. The preparation method is simple in operation, short in man hour, high in yield and suitable for industrialized production.
Owner:BEIJING JIMEITANG MEDICINE RES CO LTD
Preparation method of tebipenem pivoxil
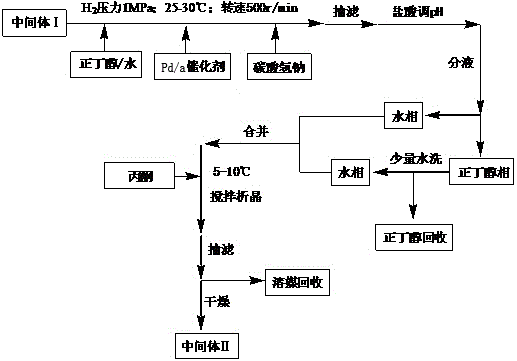
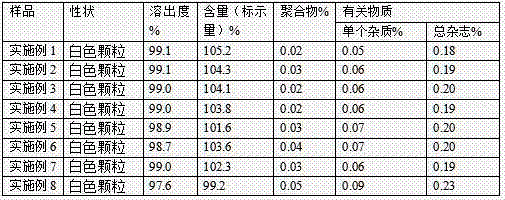
The invention provides a preparation method of tebipenem pivoxil, and relates to the technical field of pesticide synthesis. The preparation method of the tebipenem pivoxil comprises the following steps that 1-(4,5-dihydro-2-thiazolyl) azetidine-3-thiol hydrochloride and 1 beta-methyl vinyl phosphate are used as raw materials to take a reaction under the existence of diisopropylethylamine, and an acetonitrile water solution is used for washing to obtain an intermediate I; the intermediate I, an n-butyl alcohol water solution, a palladium-carbon catalyst and sodium bicarbonate take a mixed reaction, and treatment is performed to obtain an intermediate II; the intermediate II and chloromethyl pivalate take a reaction through phase transfer catalyst catalysis under the existence of diisopropylethylamine and dimethylformamide to obtain an intermediate III; the intermediate III and a sodium bicarbonate water solution are mixed, ethyl acetate is added, and reaction and refining are performed to prepare the tebipenem pivoxil. The preparation method has the advantages that the purity and the yield of the intermediates are obviously improved; the purity of the final product of the tebipenem pivoxil reaches 99.21 to 99.78 percent; the yield reaches 88.7 to 92.1 percent.
Owner:HENAN QUANYU PHARMA CO LTD
Tebipenem pivoxil granule and preparation method thereof
InactiveCN105963261AImprove stabilityProperly mask the bitternessAntibacterial agentsOrganic active ingredientsHigh humidityDissolution
The invention provides a tipipenem pectin granule and a preparation method thereof, and relates to the technical field of medicine and its preparation. A tipipenem granule, comprising the following raw materials in parts by weight: 600 to 700 parts of tipipenem, 3500 to 4500 parts of diluent, 40 to 50 parts of hydroxypropyl cellulose, and 455 to 470 parts of coating agent I 380-400 parts of coating agent II, the coating agent I is composed of ethyl cellulose suspension and talcum powder at a weight ratio of 10-20:1, the coating agent II is composed of gastric-soluble acrylic resin, Talc powder, hydroxypropyl methylcellulose and aspartame are composed in a weight ratio of 25-40:1-3:1:1.5-2.5. The tipipenem ester granule of the present invention uses tipipenem ester as the main drug, strictly screens and controls the ingredients of the excipients, optimizes the proportion, has stable drug efficacy under high temperature and high humidity environment, safe quality, high dissolution rate and excellent process. Easy to control and suitable for industrial production.
Owner:HENAN QUANYU PHARMA CO LTD
Tebipenem pivoxil industrial preparation method
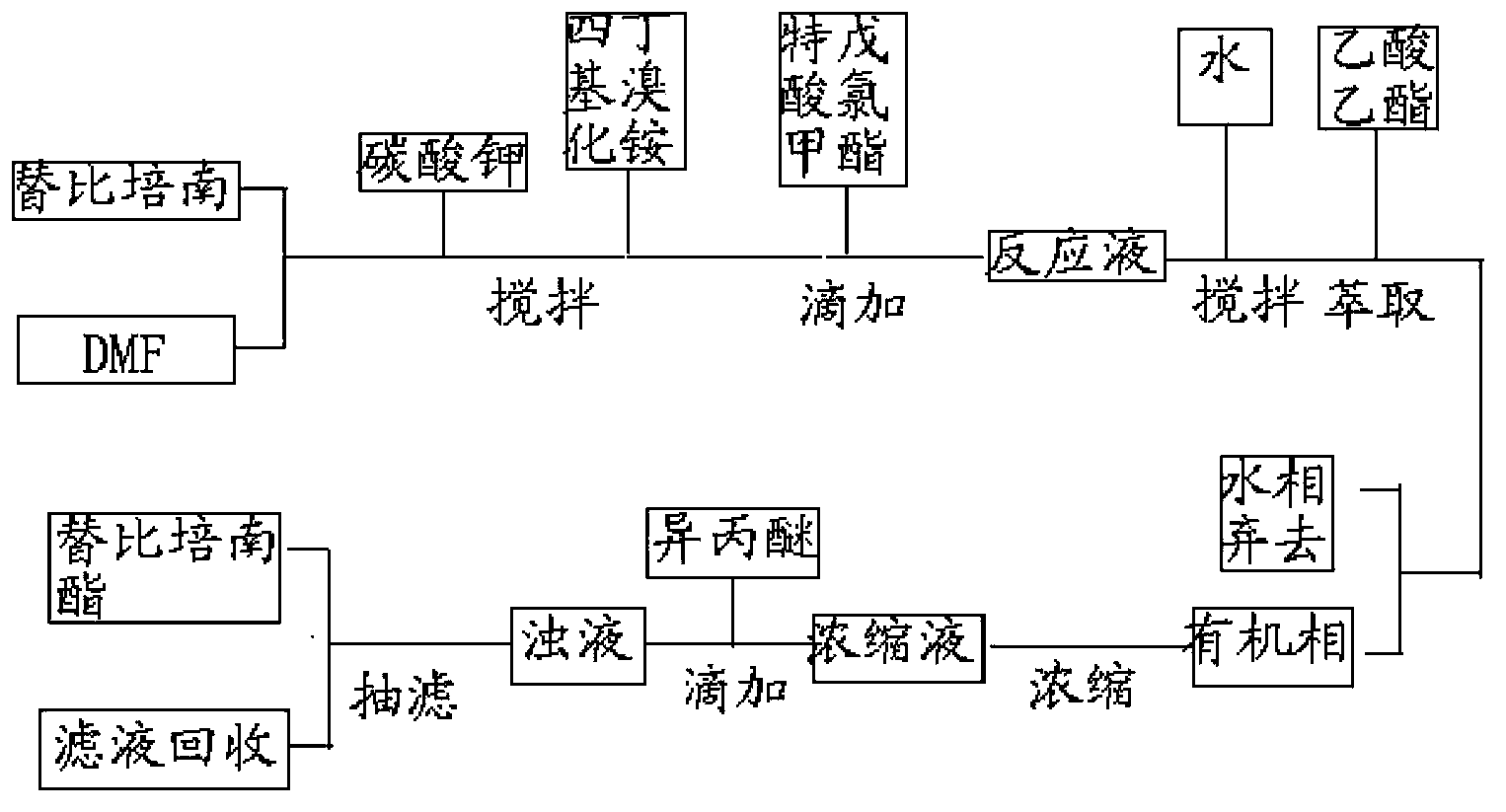
The invention relates to the field of medical chemistry, and specifically relates to a tebipenem pivoxil industrial preparation method. The method comprises the following steps: tebipenem, a solvent I, salt, and a phase-transfer catalyst are mixed, and a salt-forming reaction is carried out under normal temperature; chloromethylpivalate is added under a same temperature, and an esterification reaction is carried out; when the reaction is finished, extraction and concentration are carried out; a solvent II is dropped for crystallization; and filtering is carried out, such that tebipenem pivoxil is obtained. With the method, operation is simple, and complicated operations of pH regulation and repeated extraction are avoided. Post-treatment solvent dose is low, such that resource is saved, and environment pollution is reduced. Chloromethylpivalate property is stable, and normal-temperature reaction requirement is low. Product yield is high, and a maximal yield can be higher than 88%. The purity of synthesized tebipenem pivoxil can be higher than 99.7%. Without refining, the obtained tebipenem pivoxil can be used for preparing medicine preparations as a raw material medicine satisfying medical requirements, and the medicine preparation can be safely used by patients. The method is suitable for industrial productions.
Owner:LUNAN PHARMA GROUP CORPORATION
Synthetic method for Tebipenem Pivoxil polymer impurity
The invention provides a synthetic method for Tebipenem Pivoxil polymer impurity P8. The method employs a ring opening impurity P9 of Tebipenem and Tebipenem Pivoxil as a starting material, the starting material is subjected to an etherification reaction and an esterification reaction respectively, the obtained products are subjected to a condensation reaction and esters are formed; then t-butyl dimethyl silicon group protecting group is removed, after recrystallization purification, the target compound with a purity of being more than 90% is obtained. The target compound can be employed as a reference substance and used for qualitative and quantitative research of polymer impurities in Tebipenem Pivoxil quality research, thus contents of raw material Tebipenem Pivoxil related substances can be controlled, and quality of Tebipenem Pivoxil active pharmaceutical ingredients can be ensured.
Owner:上海津力药业股份有限公司
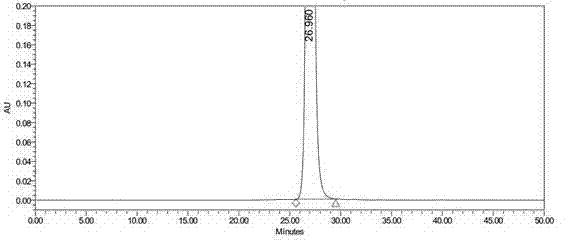
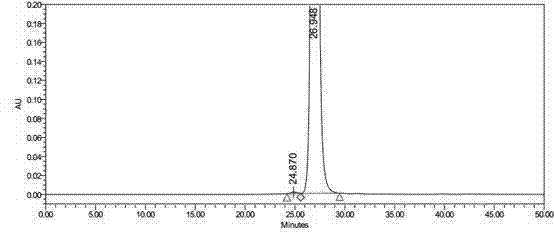
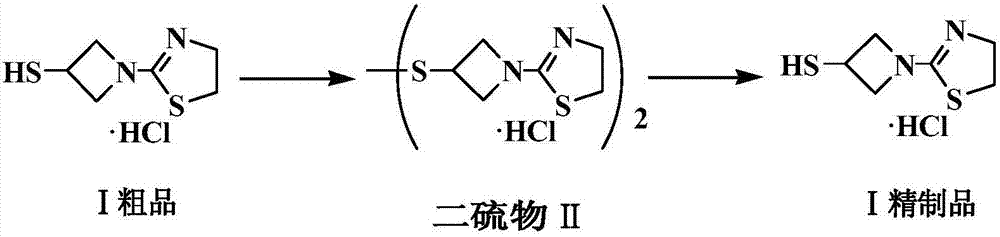
Refining method of side chain of tebipenem pivoxil
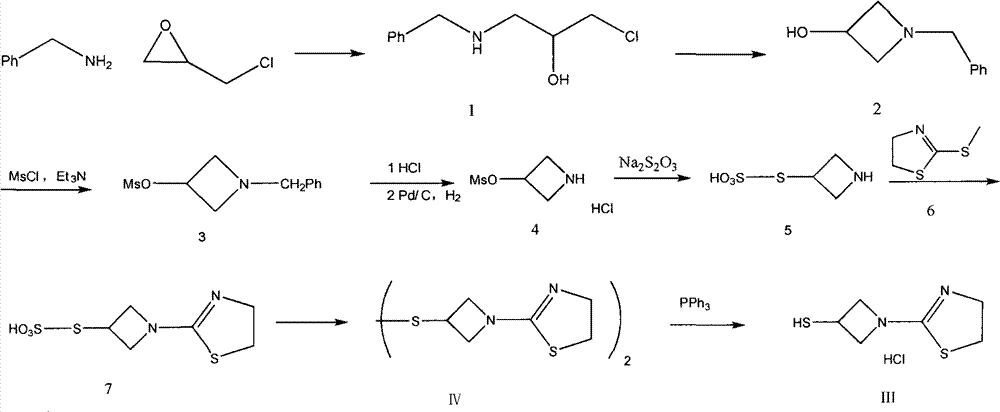
The invention discloses a refining method of a side chain of tebipenem pivoxil. The refining method comprises the following steps: oxidizing a coarse side chain of tebipenem pivoxil into a disulphide; purifying the disulphide; and then reducing the disulphide into the side chain of tebipenem pivoxil to prepare a refined product of the side chain of tebipenem pivoxil. Compared with a method of physically refining the coarse side chain of tebipenem pivoxil, the refined side chain of tebipenem pivoxil prepared by the refining method is high in yield, high in purity and stable in chemical property, and the refining method disclosed by the invention has the advantages of being simple to operate, economical and practical, suitable for industrial production and the like.
Owner:ZHEJIANG HISOAR CHUANNAN PHARMA +1
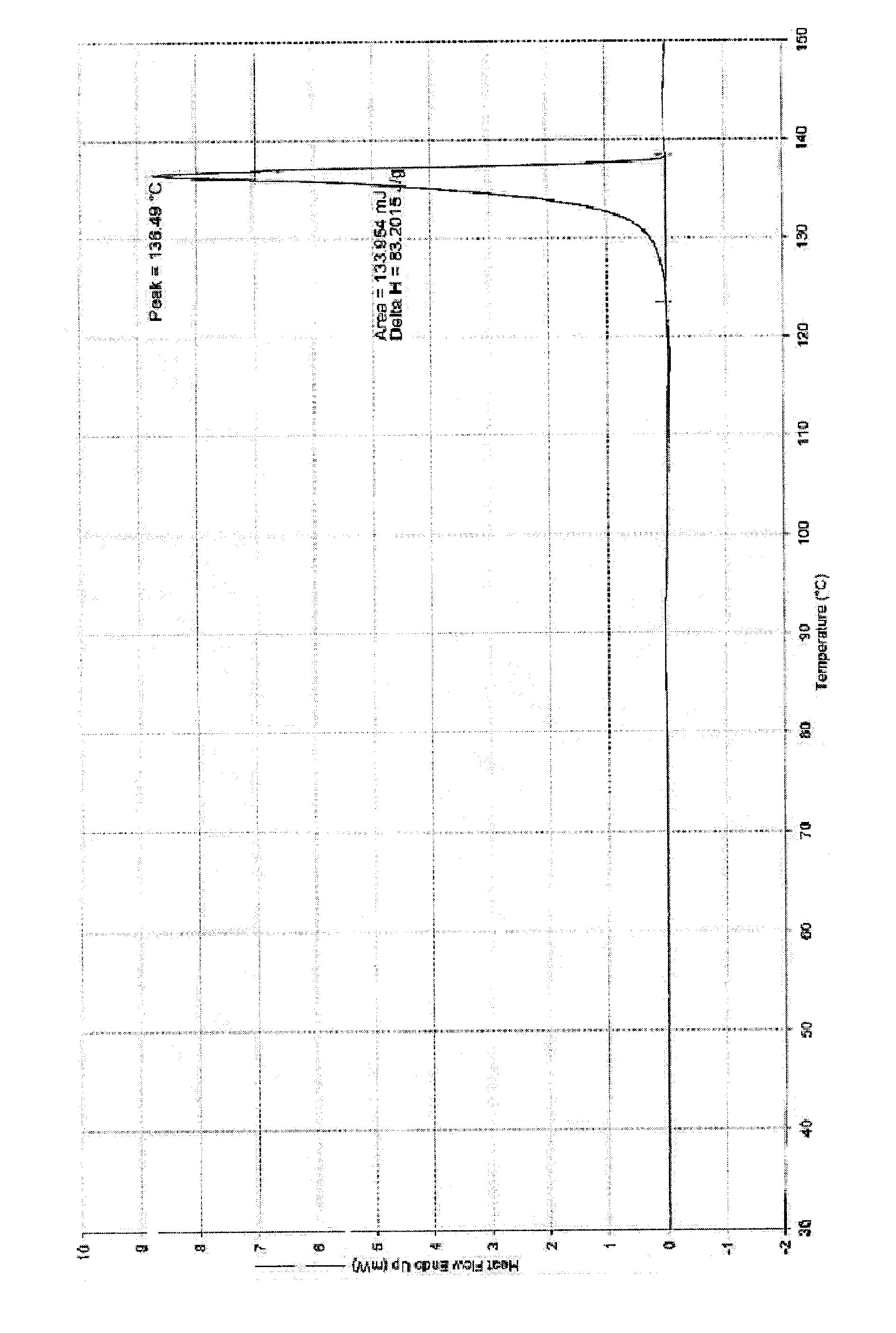
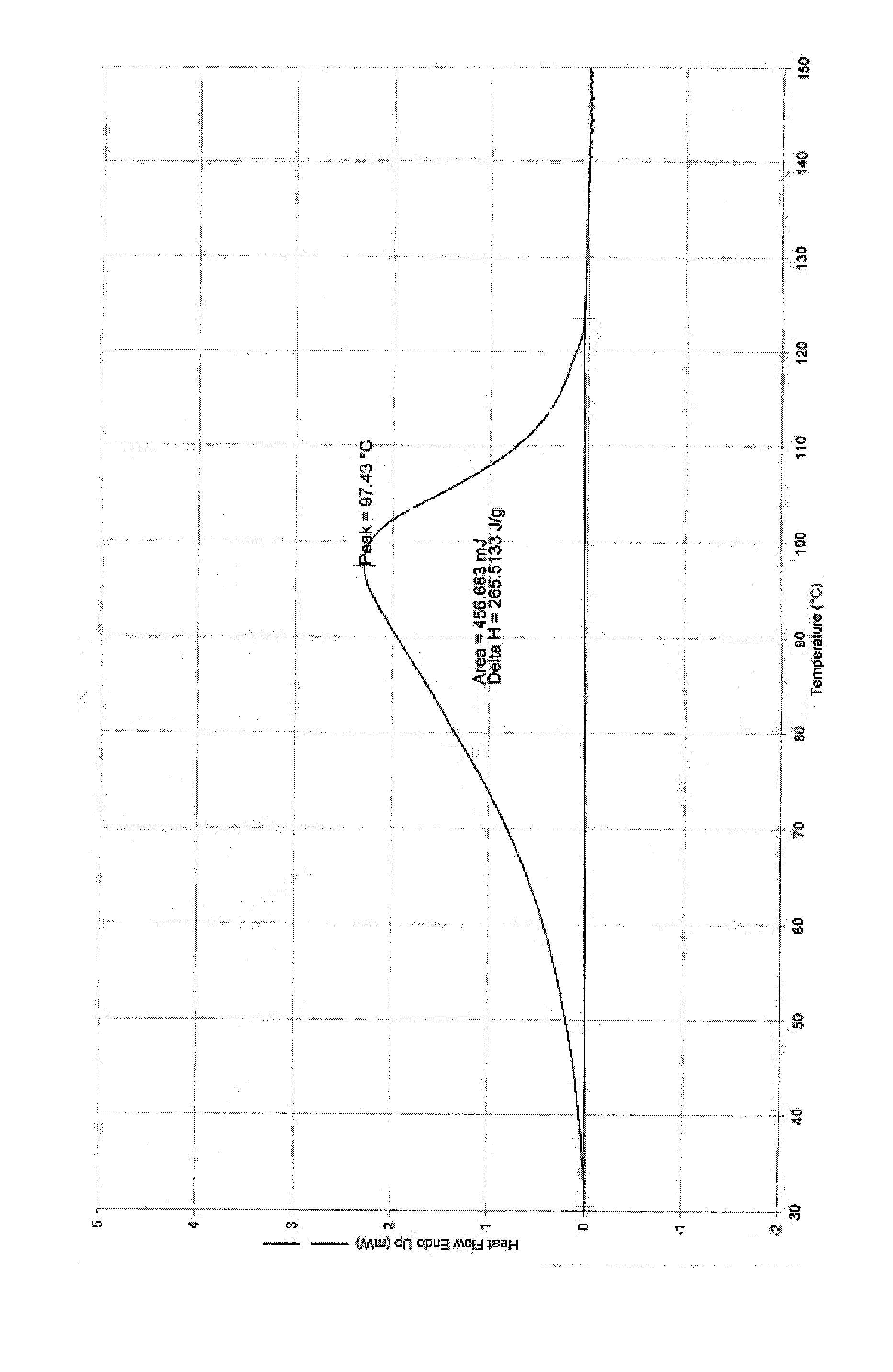
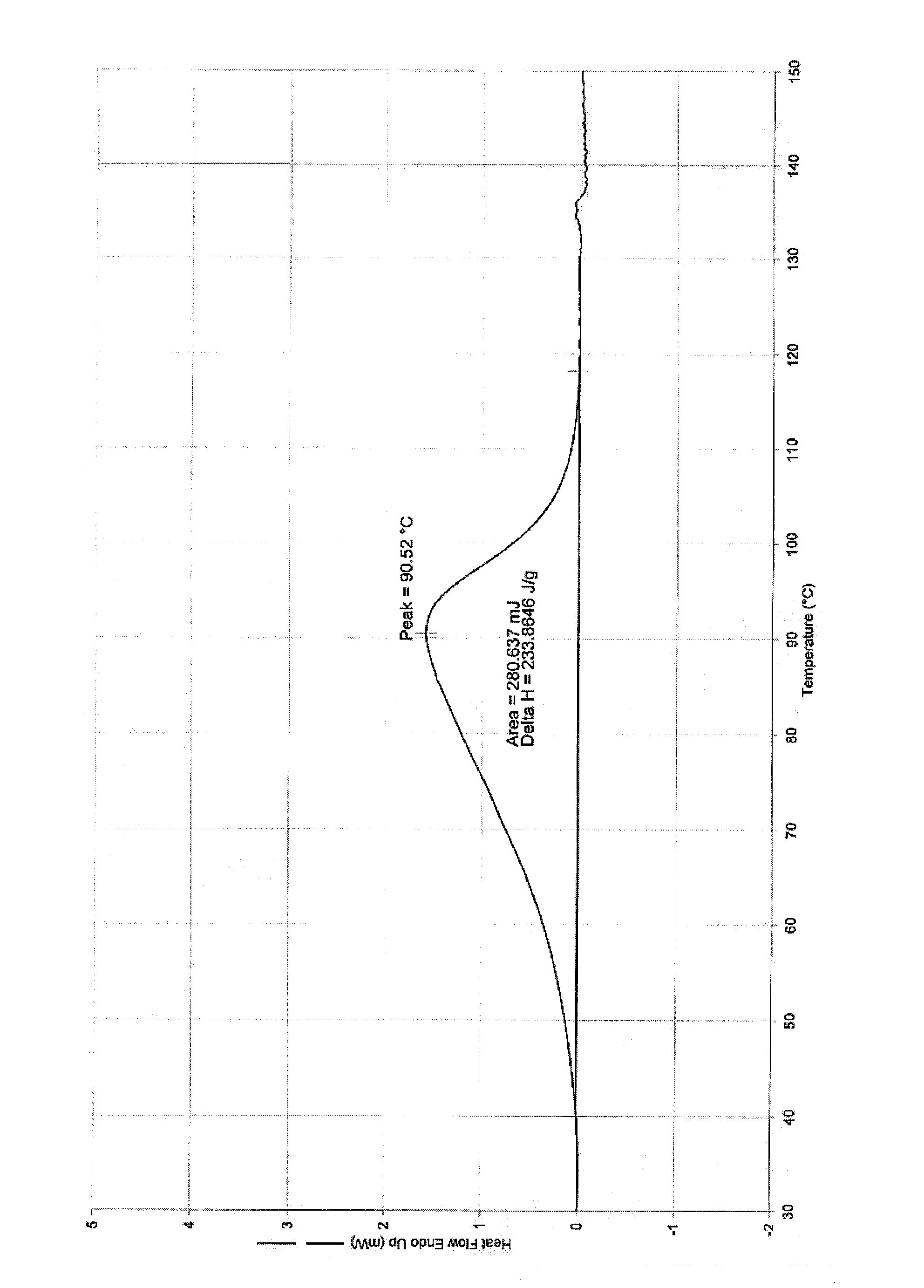
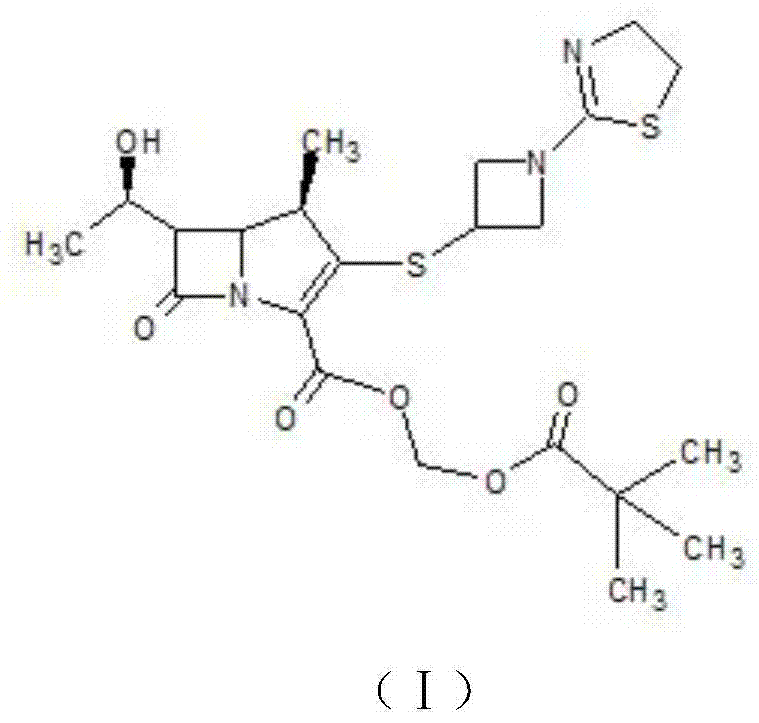
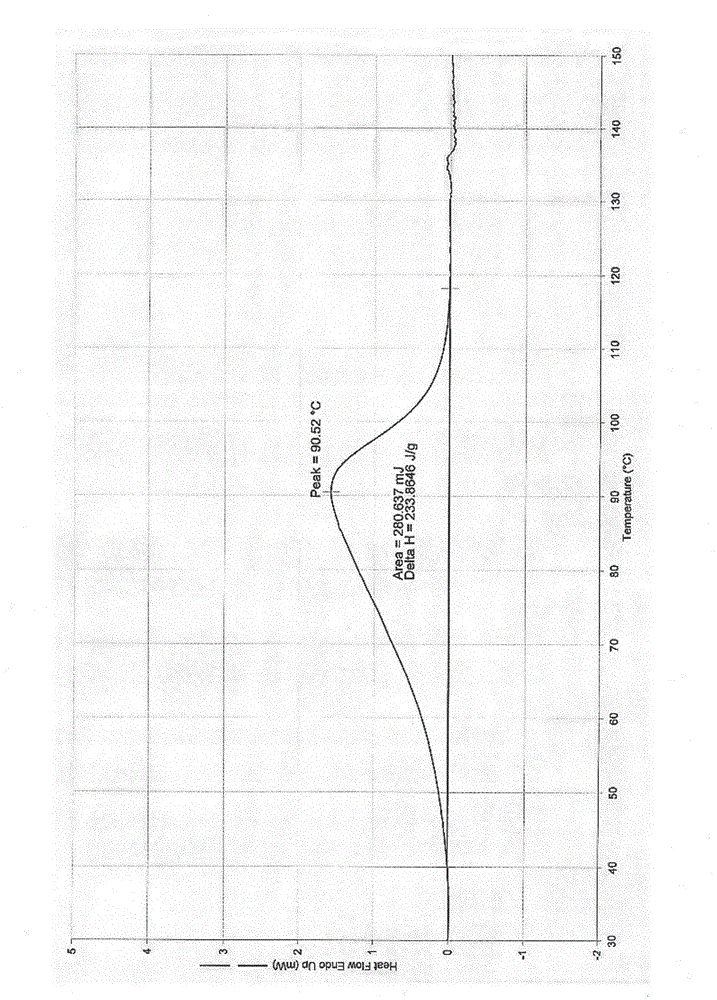
Tebipenem pivoxil crystal and preparation method thereof
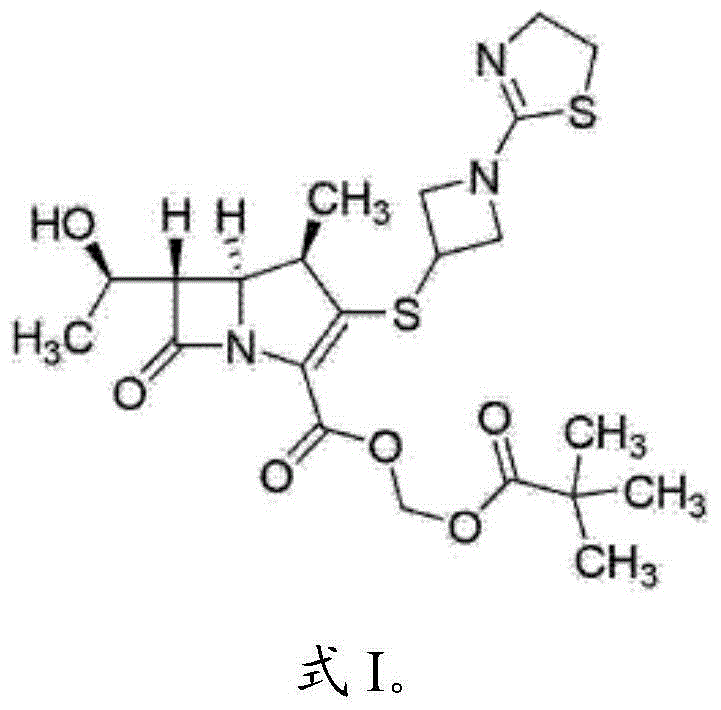
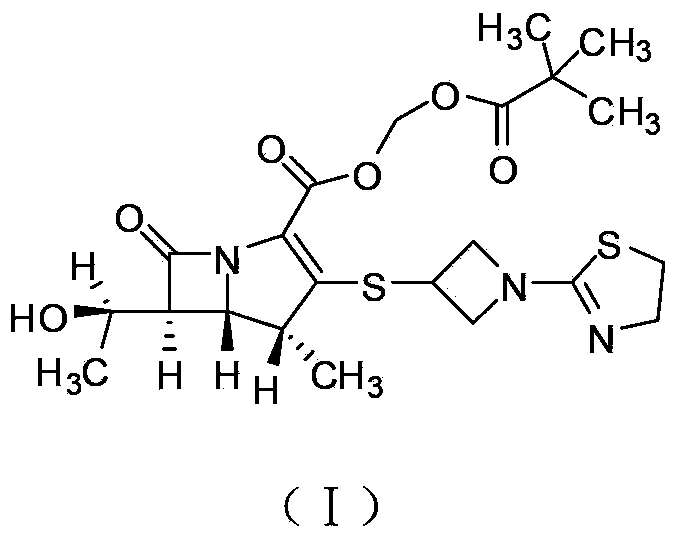
The invention relates to a tebipenem pivoxil crystal shown by a formula (I) and a preparation method thereof. The invention provides a high-purity tebipenem pivoxil crystal shown by the formula (I) and a preparation method thereof. The preparation method comprises the steps of dissolving (+)-(4R,5S,6S)-6-[(1R)-1-ethoxyl]-4-methyl-7-oxo-3-[[1-(2-thiazoline-2-yl)-3-azetidinyl]sulfo]-1-azabicyclo[3.2.0]-hepta-2-alkene-2-carboxylic acid-2-methyl trimethylacetate (tebipenem pivoxil) in a single or mixed solvent of ethanol, acetonitrile, acetic ether, acetone and n-hexane at a specified temperature, and performing recrystallization and drying to prepare the high-purity tebipenem pivoxil crystal. The high-purity tebipenem pivoxil crystal is stable in crystal form, all indexes of the crystal meet requirements, and the crystal is convenient to produce and store.
Owner:高瑞耀业(北京)科技有限公司 +1
Preparation method of tebipenem pivoxil granules
InactiveCN102824314AImprove qualityHigh dissolution rateAntibacterial agentsOrganic active ingredientsChemical synthesisSucrose
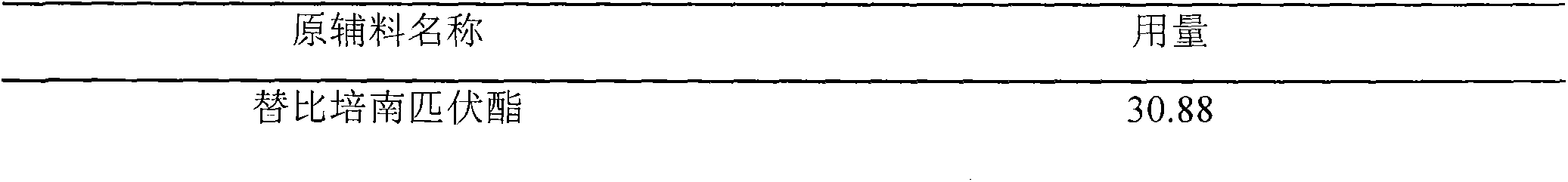
The invention relates to the field of chemical synthesis, and discloses a preparation method of tebipenem pivoxil granules. The preparation method comprises the following steps of: grinding the following raw materials in parts by weight: 32.44 parts of tebipenem pivoxil, 31.25 parts of microcrystalline cellulose, 183.56 parts of sucrose, 2.5 parts of aspartame and 0.25 part of red iron oxide; and mixing uniformly and feeding the mixture into a dry granulator for granulation. The method disclosed by the invention adopts four accessories with wide source and better compatibility with the primary medicine tebipenem pivoxil to prepare new granules; and the prepared granules can ensure better dissolution rate, relatively low content of impurities and polymers and the like under the condition of relatively few accessories, have good quality and can be widely applied to clinical treatment.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Tebipenem pivoxil granules
InactiveCN102836130AImprove qualityHigh dissolution rateAntibacterial agentsOrganic active ingredientsBiotechnologyChemical synthesis
The invention relates to the field of chemical synthesis, and discloses a tebipenem pivoxil granule. The granule disclosed by the invention consists of 32.44 weight parts of tebipenem pivoxil, 31.25 weight parts of microcrystalline cellulose, 183.56 weight parts of cane sugar, 2.5 weight parts of aspartame and 0.25 weight parts of red iron oxide. According to the invention, the novel granule is obtained by adopting four auxiliary materials which have varieties of sources and are well compatible with the main drug, namely the tebipenem pivoxil. The granule disclosed by the invention has the advantages that higher dissolution rate, lower impurity rate and lower polymerizability can be still guaranteed even under the circumstance that less auxiliary materials are adopted, so the granule has higher quality and can be widely applied to clinical treatment.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
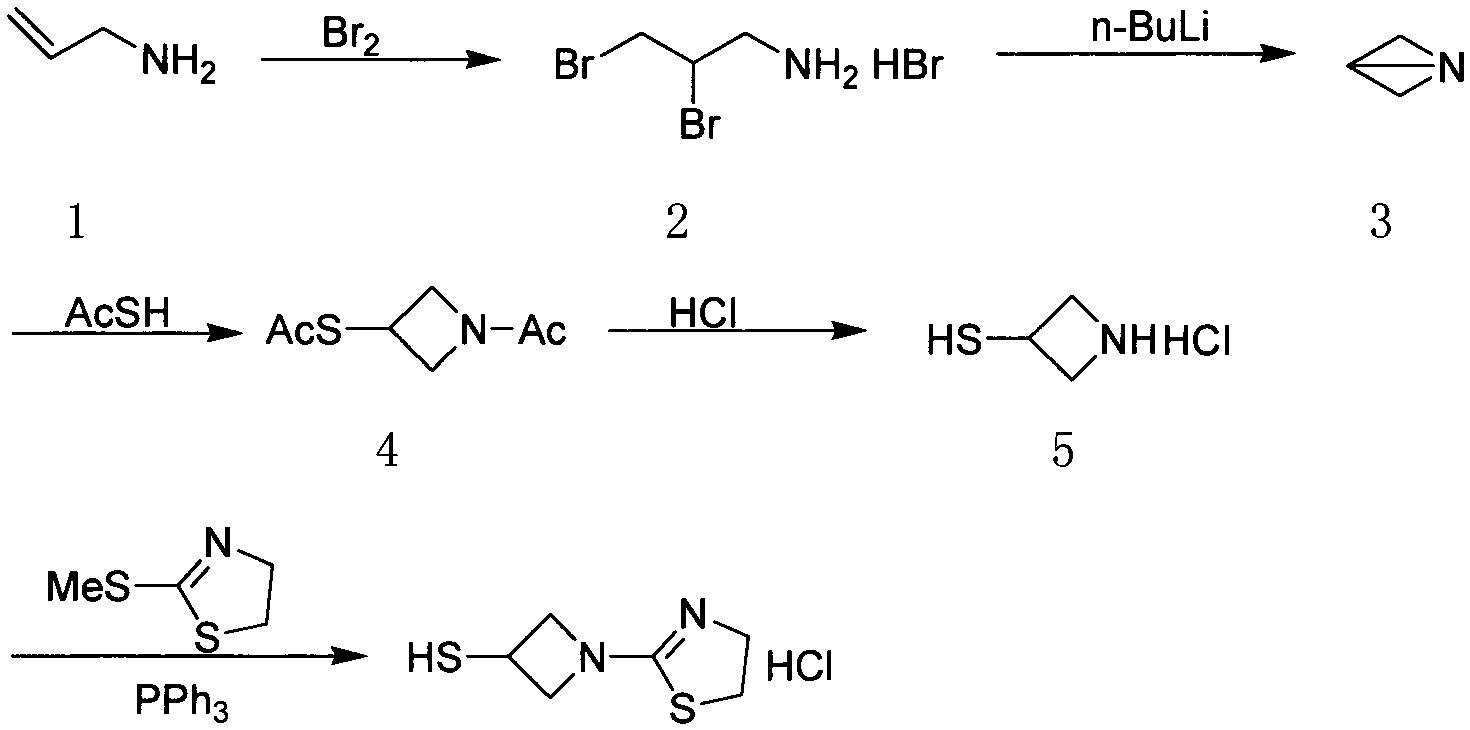
Novel synthetic method of tebipenem pivoxil side chain
ActiveCN104292222AReduce pollutionRaw materials are cheap and easy to getOrganic chemistryBulk chemical productionSide chainTEBIPENEM PIVOXIL
The invention discloses a novel synthetic method of a tebipenem pivoxil side chain. The method employs cheap and easily available raw material, has simple experimental operations, high overall yield and is applicable to industrialized production.
Owner:CHANGZHOU RUIBO BIO TECH
Oral preparation containing tebipenem pivoxil and preparation method thereof
ActiveCN107737107AReduce direct contactMask bitternessAntibacterial agentsOrganic active ingredientsSolubilityDissolution
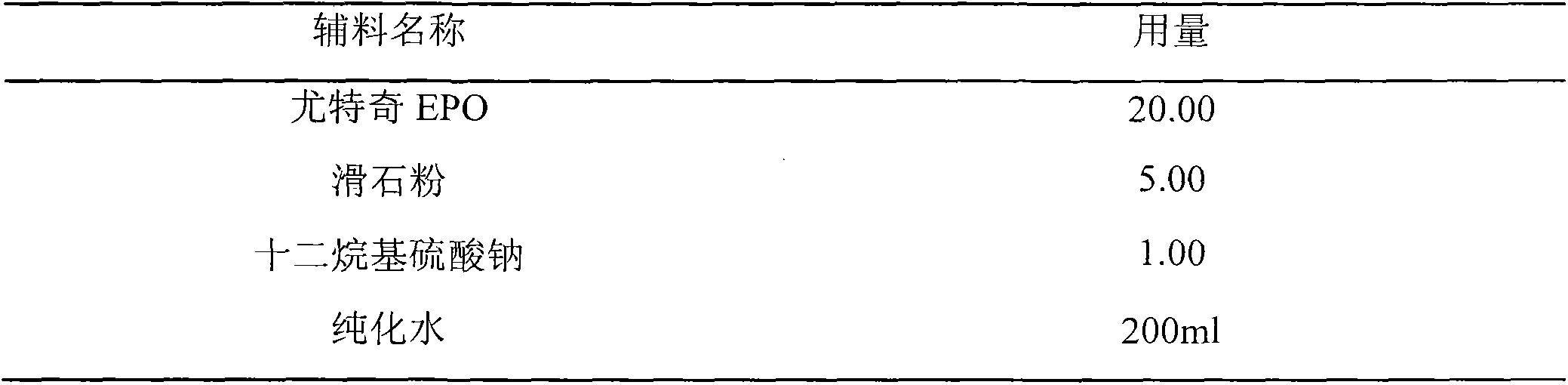
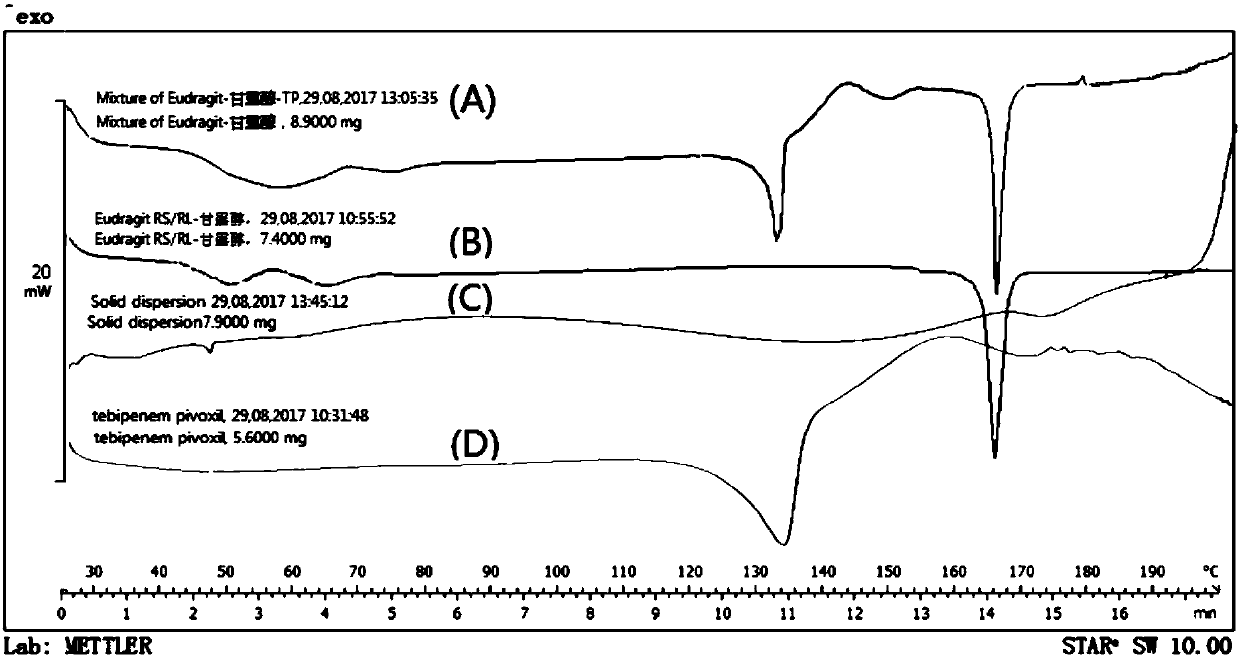
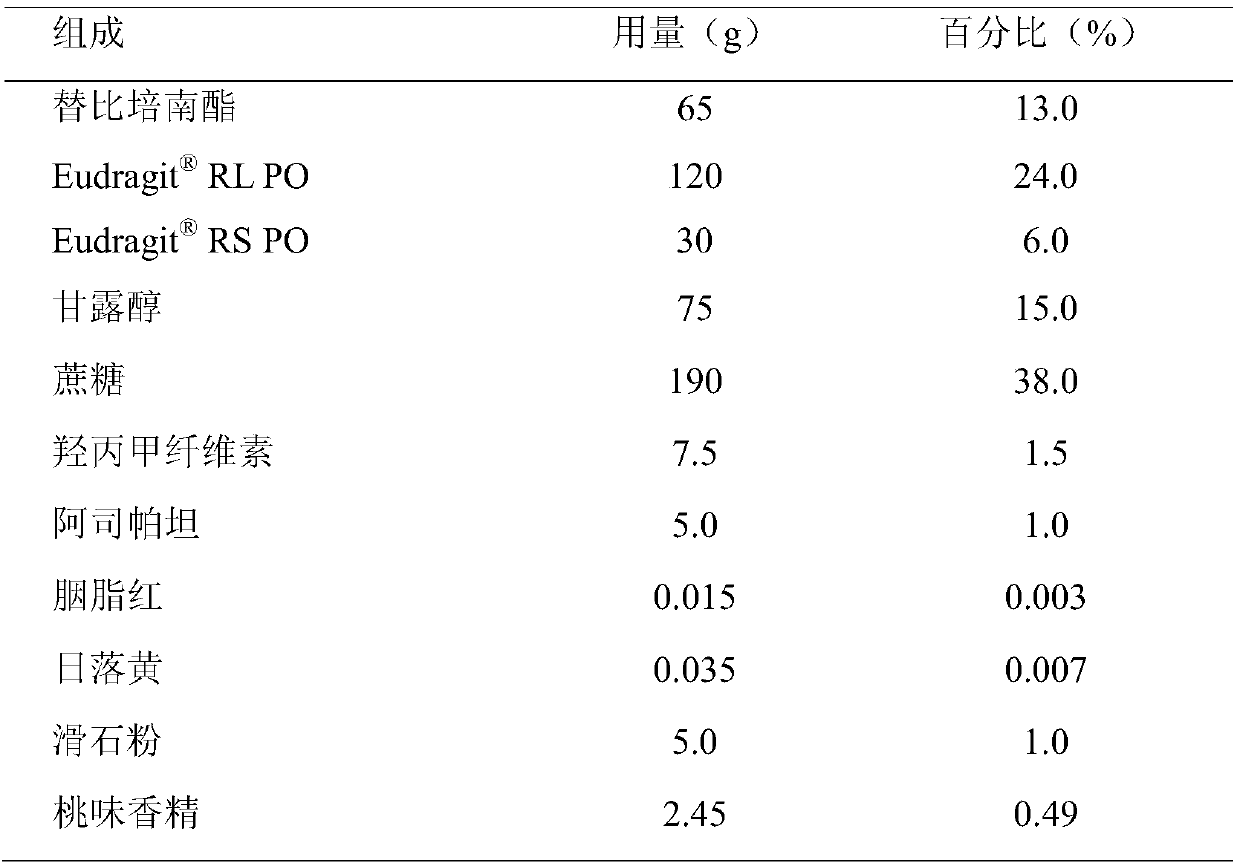
The invention belongs to the technical field of pharmaceutical preparations and particularly relates to an oral preparation containing tebipenem pivoxil and a preparation method thereof. The pharmaceutical preparation is prepared by the following steps: preparing a solid dispersion from an active ingredient tebipenem pivoxil; then crushing and screening the solid dispersion; and preapring the oralpreparation containing tebipenem pivoxil with proper diluent, adhesive, sweetening agent, lubricant, pigments and essence according to a conventional granulating process. The oral preparation containing tebipenem pivoxil prepared by the method covers strong bitterness of the drug effectively, so that the administrating compliance of a child patient is improved extremely; the dissolution rate andthe solubility of the drug are improved remarkably, and quick release of the drug is accelerated; the drug stability is increased, and the physical aging problem of conventional coating treatment is avoided; and the process is simple to operate, good in repeatability and suitable for industrial production.
Owner:北京达因高科儿童药物研究院有限公司 +1
Method for preparing Tebipenem pivoxil
The invention relates to the technical field of medicines and particularly relates to a method for preparing Tebipenem pivoxil. The method for preparing the Tebipenem pivoxil, provided by the invention, at least comprises the following steps: (1) condensation reaction; (2) hydrogenation reaction; (3) esterification reaction; and (4) purification and refining.
Owner:科兴生物制药股份有限公司
Preparation method of antibacterial agent
ActiveCN103012406ARaw materials are easy to getSimple process routeOrganic chemistryBulk chemical productionSodium iodideCarboxylic acid
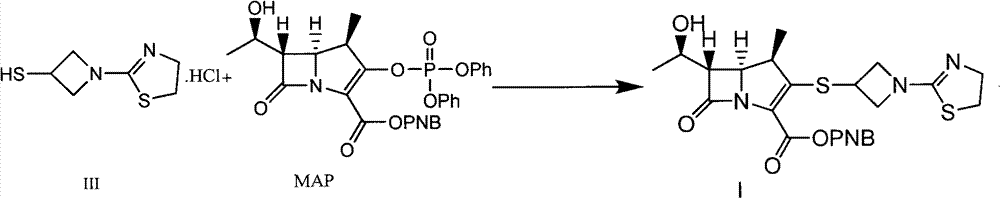
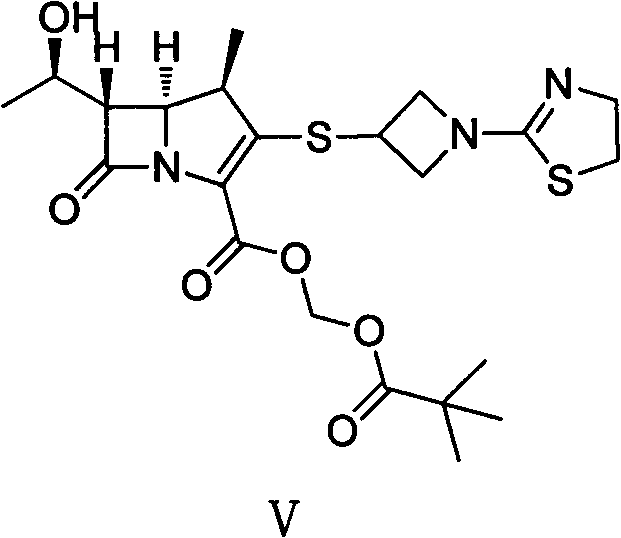
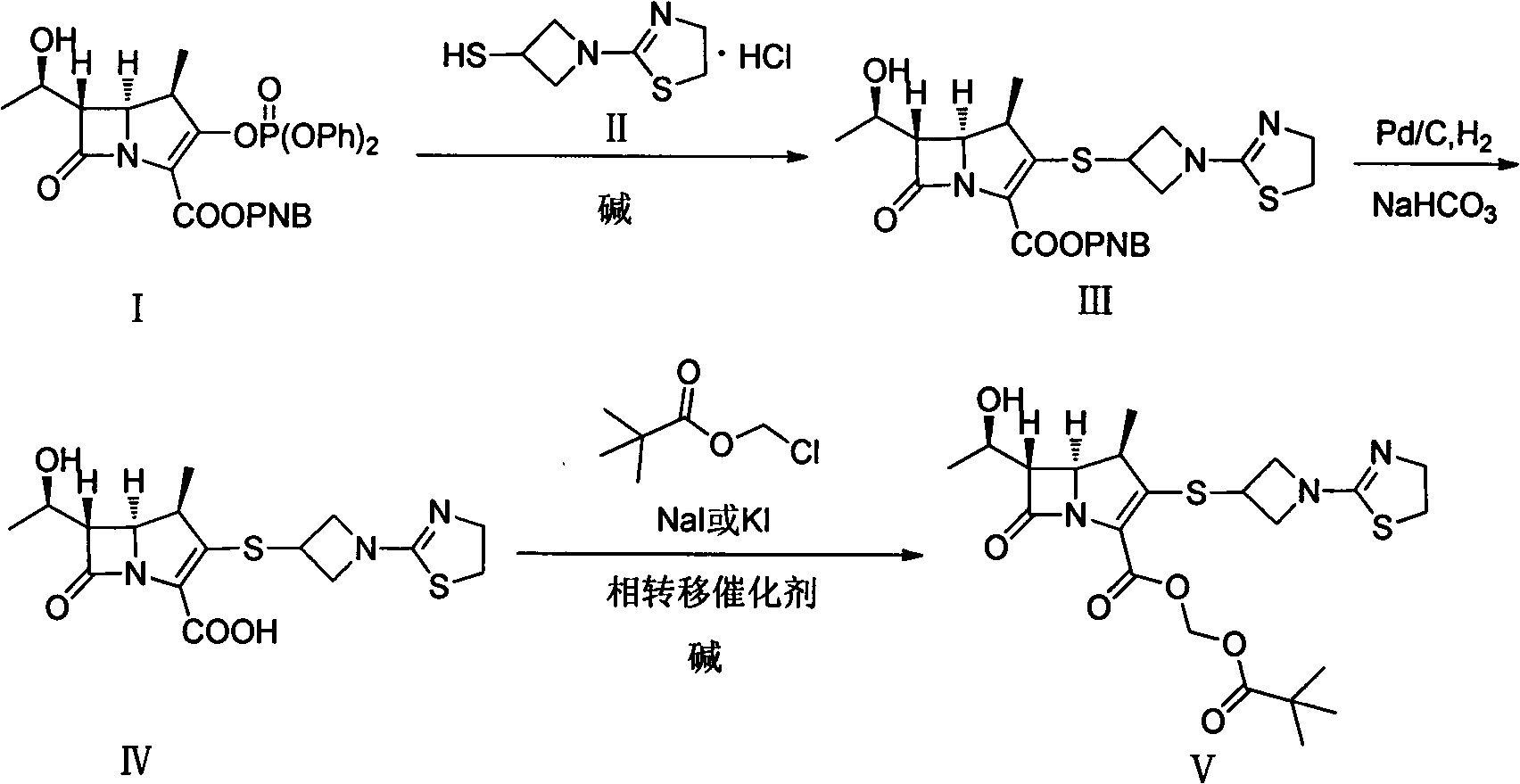
The invention discloses a preparation method of an antibacterial medicament tebipenem pivoxil. (4R,5R,6S)-3-((diphenyl-phosphoroso-carbonyl)oxyl)-6-((R)-1-ethoxyl)-4-methyl-7-carbonyl-1-azabicyclo[3.2.0]heptane-2-alkenyl-2-carboxylic acid p-nitro ester(6-MAP(I)) serving as a raw material reacts with 1-(4,5-dihydro-2-thiazolinyl)-3-sulfydryl azetidine hydrochloride (II) in the presence of alkali to obtain (4R,5S,6S)-3-((1-(4,5-dihydro-2-thiazolinyl)-3-azetidine) sulfenyl)-6-((R)-1-ethoxy)-4-methyl-7-oxygen-1-azabicyclo[3.2.0]heptane-2-alkenyl-2-carboxylic acid p-nitro ester (III); the protecting group of the compound (III) is removed under the catalytic hydrogenation condition to obtain (4R,5S,6S)-3-((1-(4,50dihydro-2-thiazolinyl)-3-azetidine)sulfenyl)-6-((R)-1-ethoxy)-4-methyl-7-oxygen-1-azabicyclo[3.2.0]heptane-2-alkenyl-2-carboxylic acid (IV); and the compound (IV) reacts with chloromethyl pivalate and sodium iodide or potassium iodide in the presence of alkali to obtain tebipenem pivoxil (V). According to the preparation method, the selected initial raw materials are low in price and easily available, so that the synthesizing route is simplified, the raw material utilization and total yield can be improved; and the intermediate substance obtained in the reaction is purified by using a re-crystallization method with high yield, less three wastes are generated in the reacting process, and the low cost is advantageous to industrial production.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Tebipenem pivoxil oral preparation and preparation method thereof
ActiveCN102860985BDisintegrates quicklyReduce manufacturing costAntibacterial agentsPowder deliveryActive componentPatient compliance
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Crystal of intermediate of tebipenem pivoxil and preparation method thereof
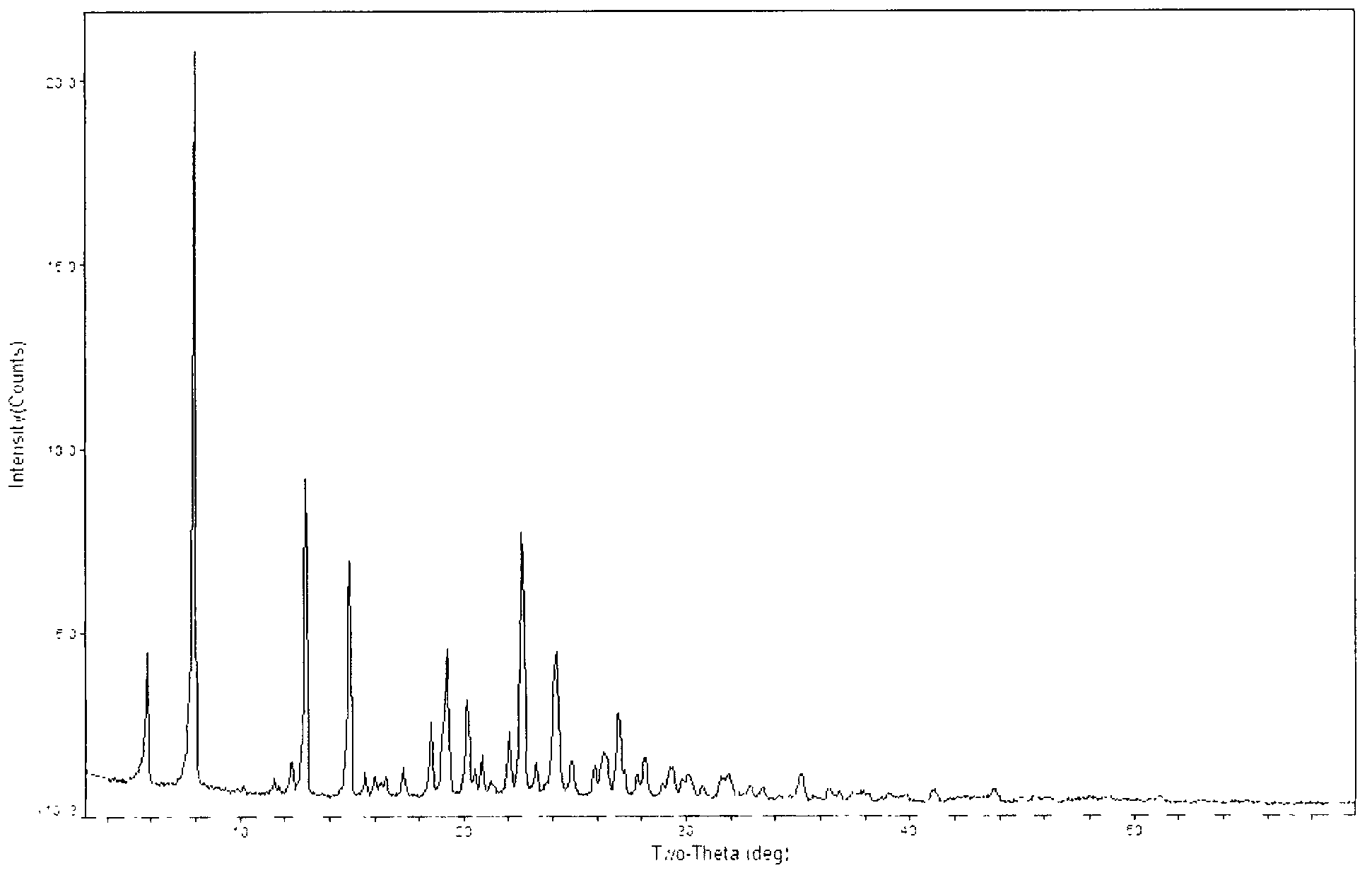
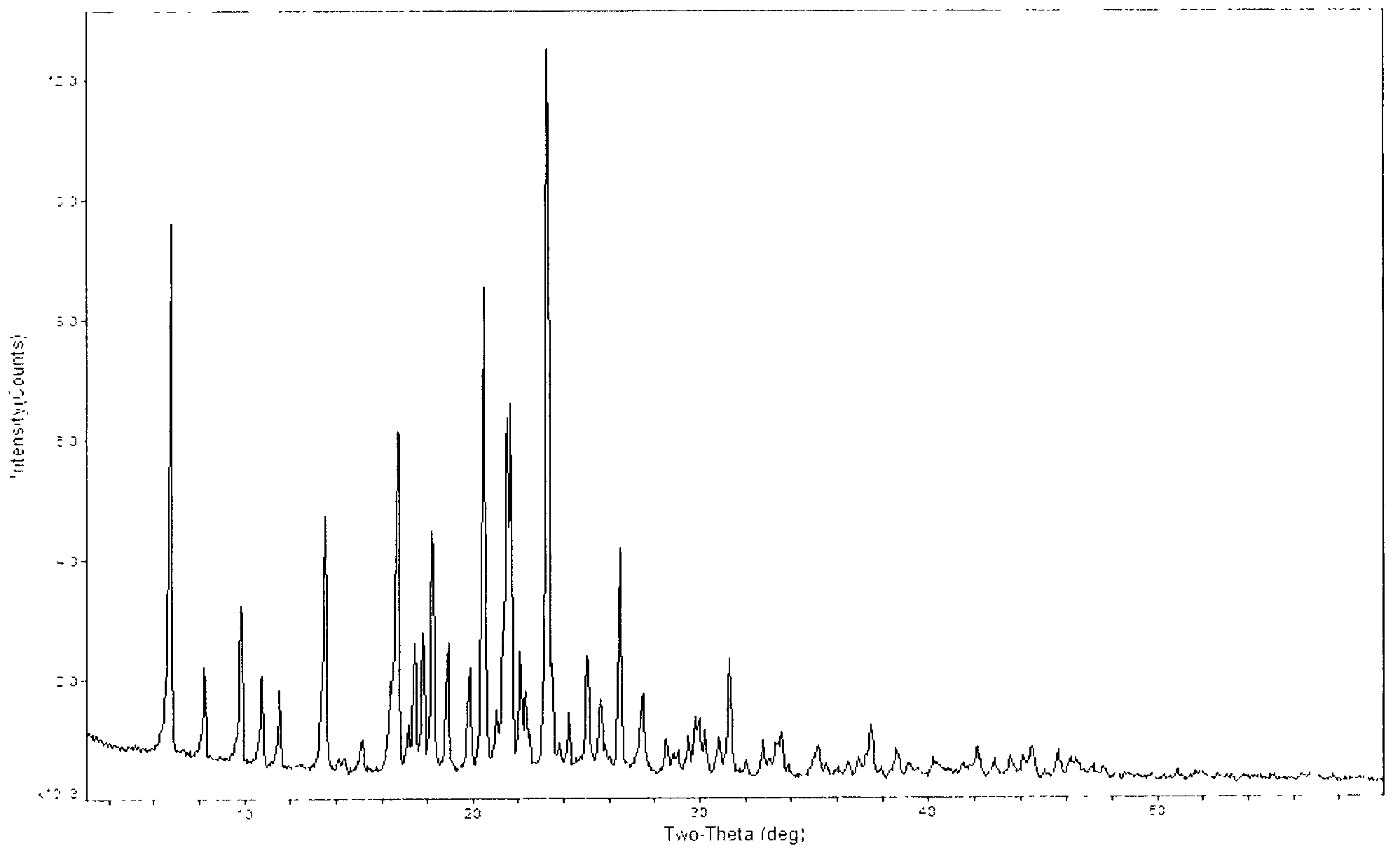
The invention relates to a crystal of an intermediate of carbapenem antibiotic tebipenem pivoxil and a preparation method thereof. The crystal type of the intermediate comprises a type A crystal of the intermediate of tebipenem pivoxil and a type B crystal of the intermediate of tebipenem pivoxil, wherein in a powder X-ray diffraction pattern, the type A crystal has main peaks when the diffraction angle 2(theta) is 5.90, 8.02, 14.93, 19.30, 22.65, 22.10, 24.16, 26.50 and 26.98; the type B crystal has main peaks when the diffraction angle 2(theta) is 6.76, 8.25, 15.13, 16.35, 17.47, 21.02, 22.31, 31.31 and 32.77. The preparation method of the crystal form mainly comprises a preparation method of the type A crystal of the intermediate and a method for preparing the type B crystal through crystal transformation of the type A crystal.
Owner:SHANDONG MINGREN FURUIDA PHARMA +1
The preparation method of tipipenem ester
The invention provides a preparation method of tebipenem pivoxil, and relates to the technical field of pesticide synthesis. The preparation method of the tebipenem pivoxil comprises the following steps that 1-(4,5-dihydro-2-thiazolyl) azetidine-3-thiol hydrochloride and 1 beta-methyl vinyl phosphate are used as raw materials to take a reaction under the existence of diisopropylethylamine, and an acetonitrile water solution is used for washing to obtain an intermediate I; the intermediate I, an n-butyl alcohol water solution, a palladium-carbon catalyst and sodium bicarbonate take a mixed reaction, and treatment is performed to obtain an intermediate II; the intermediate II and chloromethyl pivalate take a reaction through phase transfer catalyst catalysis under the existence of diisopropylethylamine and dimethylformamide to obtain an intermediate III; the intermediate III and a sodium bicarbonate water solution are mixed, ethyl acetate is added, and reaction and refining are performed to prepare the tebipenem pivoxil. The preparation method has the advantages that the purity and the yield of the intermediates are obviously improved; the purity of the final product of the tebipenem pivoxil reaches 99.21 to 99.78 percent; the yield reaches 88.7 to 92.1 percent.
Owner:HENAN QUANYU PHARMA CO LTD
Oral solid preparation of tipipenem axetil and preparation method thereof
ActiveCN103054815BAffect quality issuesEnsure safetyAntibacterial agentsOrganic active ingredientsDrug productTEBIPENEM PIVOXIL
Owner:NANJING CAVENDISH BIO ENG TECH +1
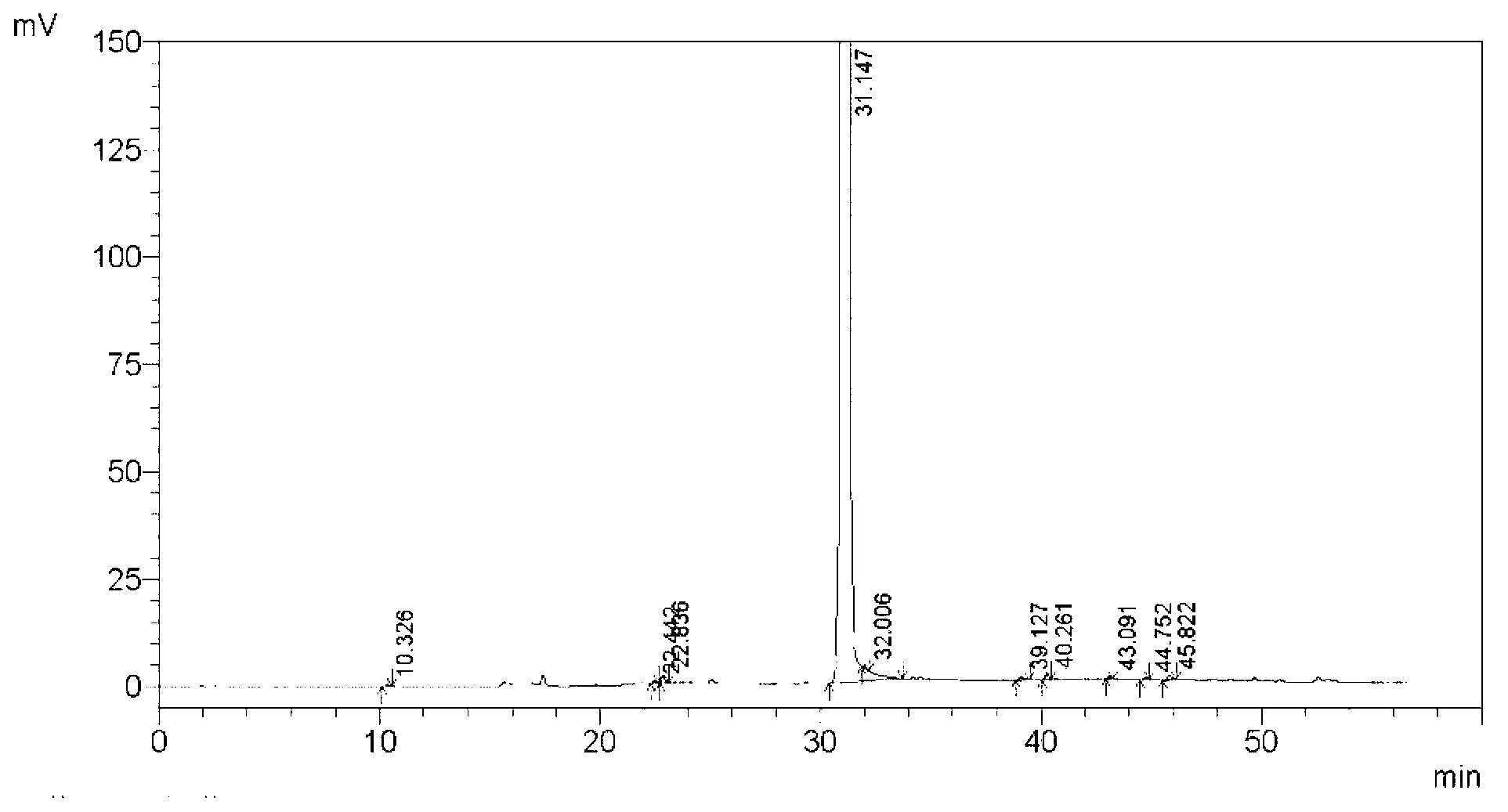
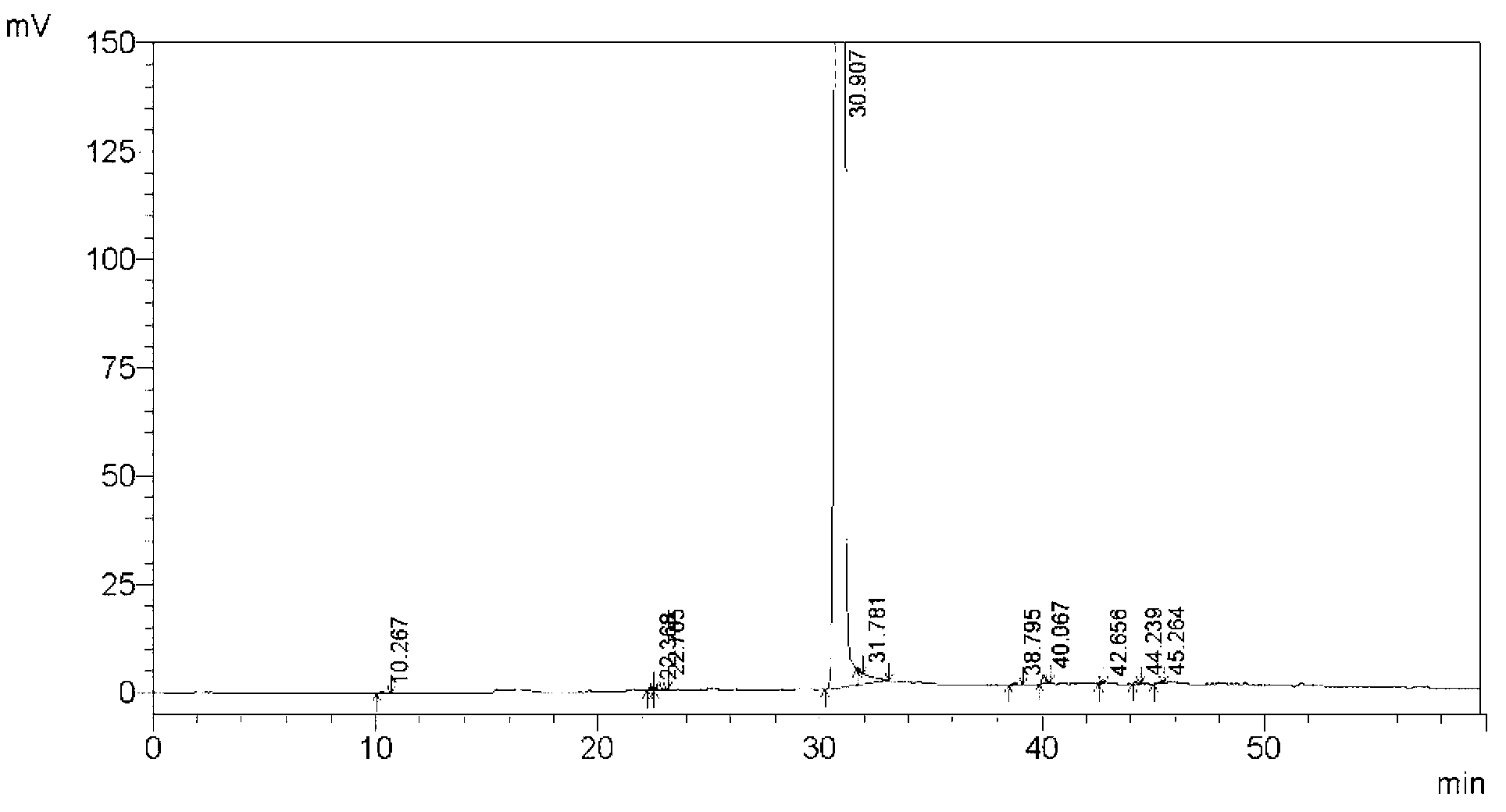
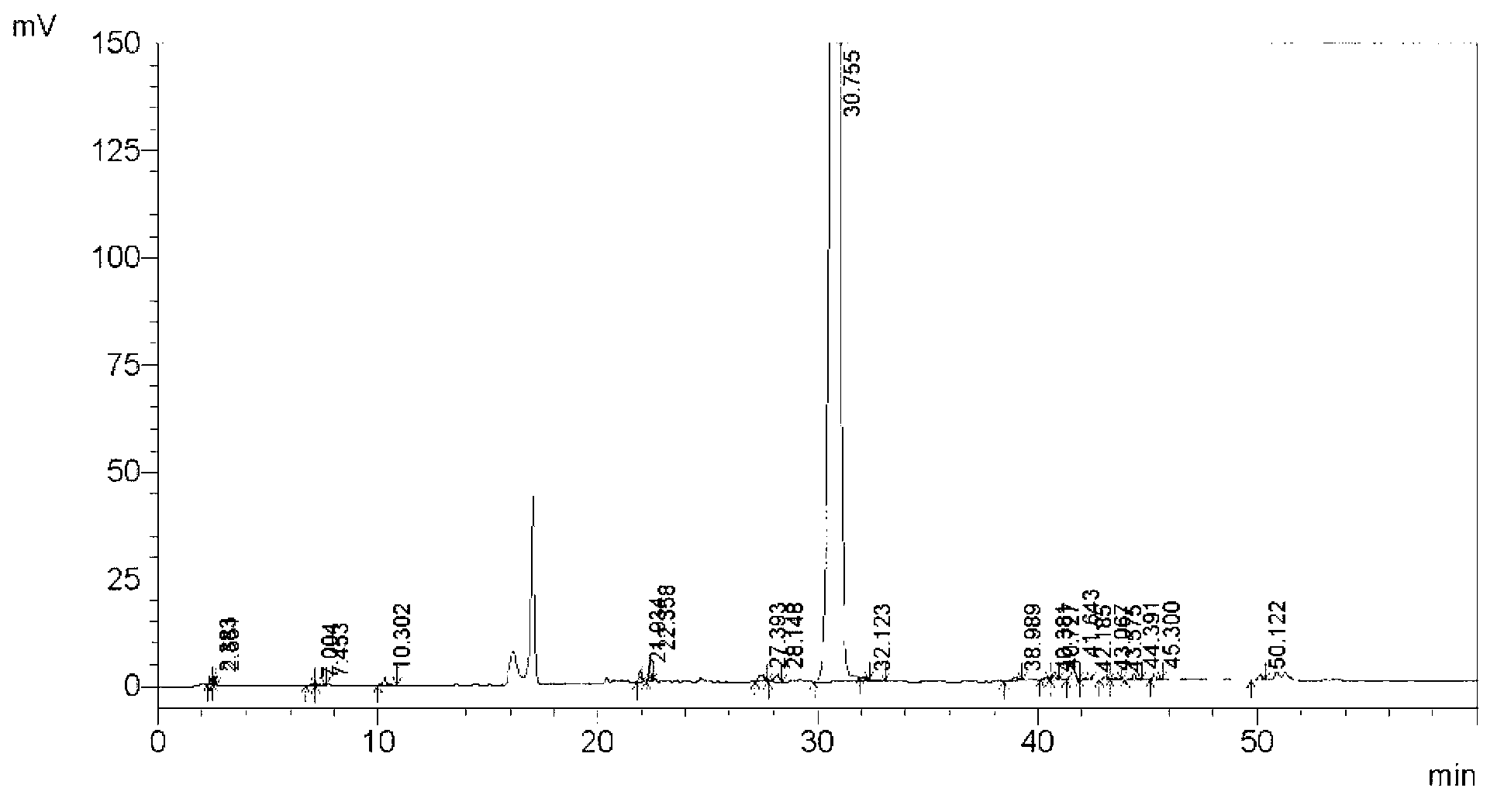
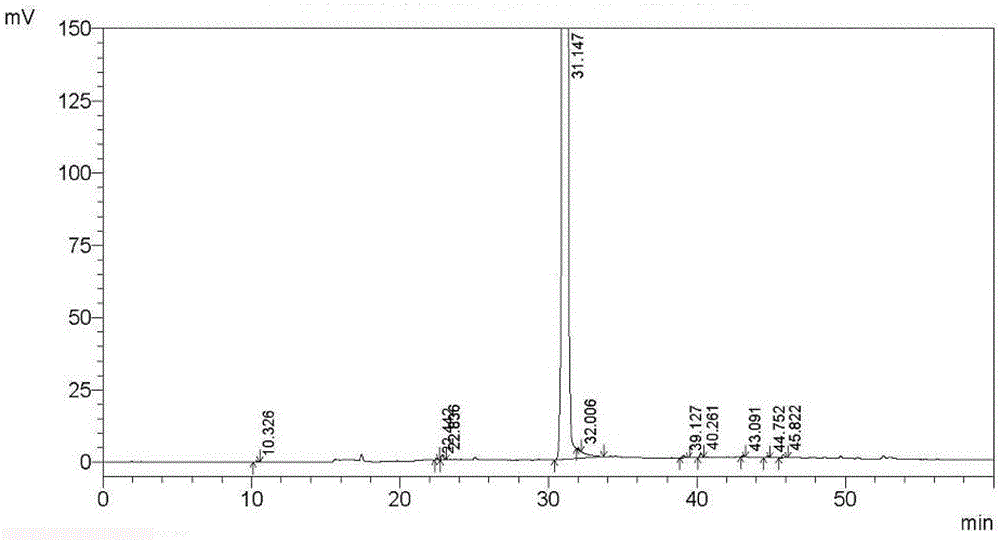
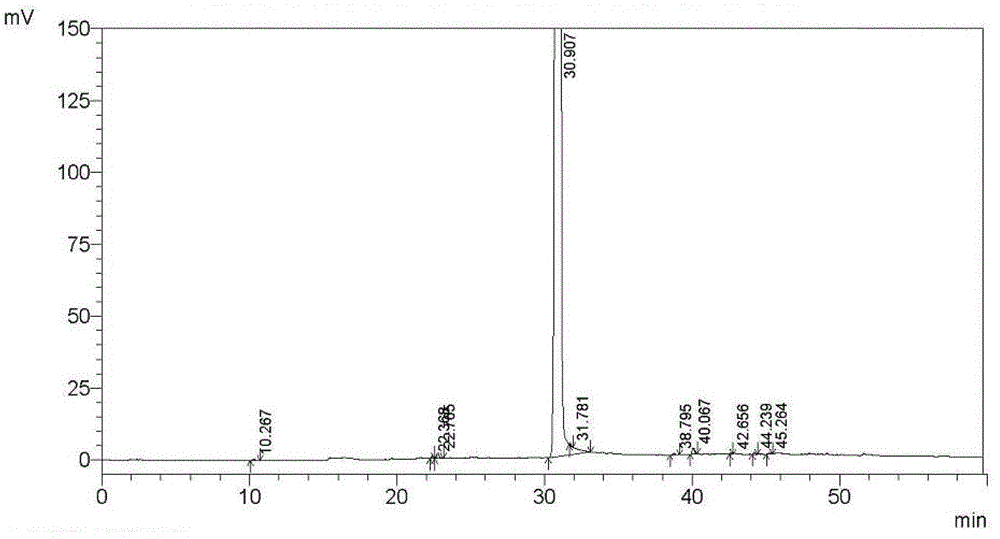
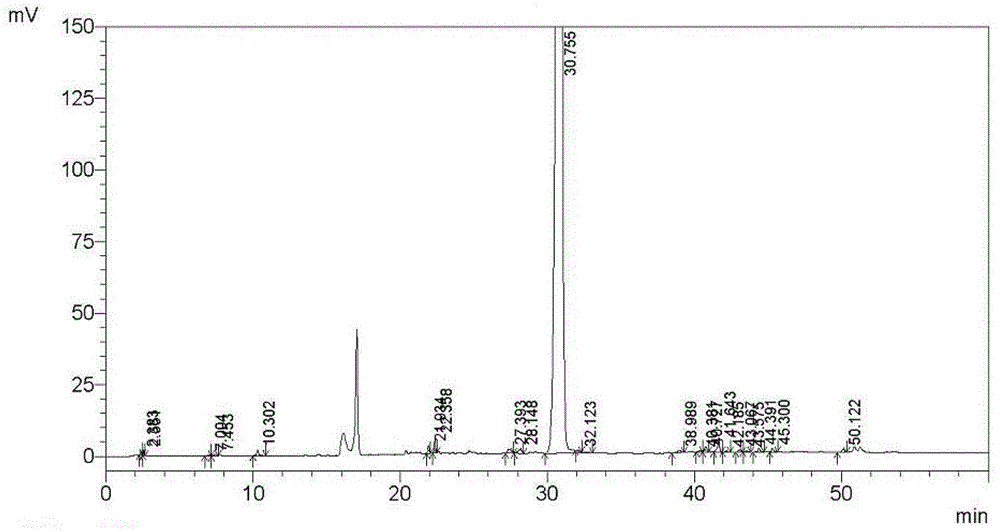
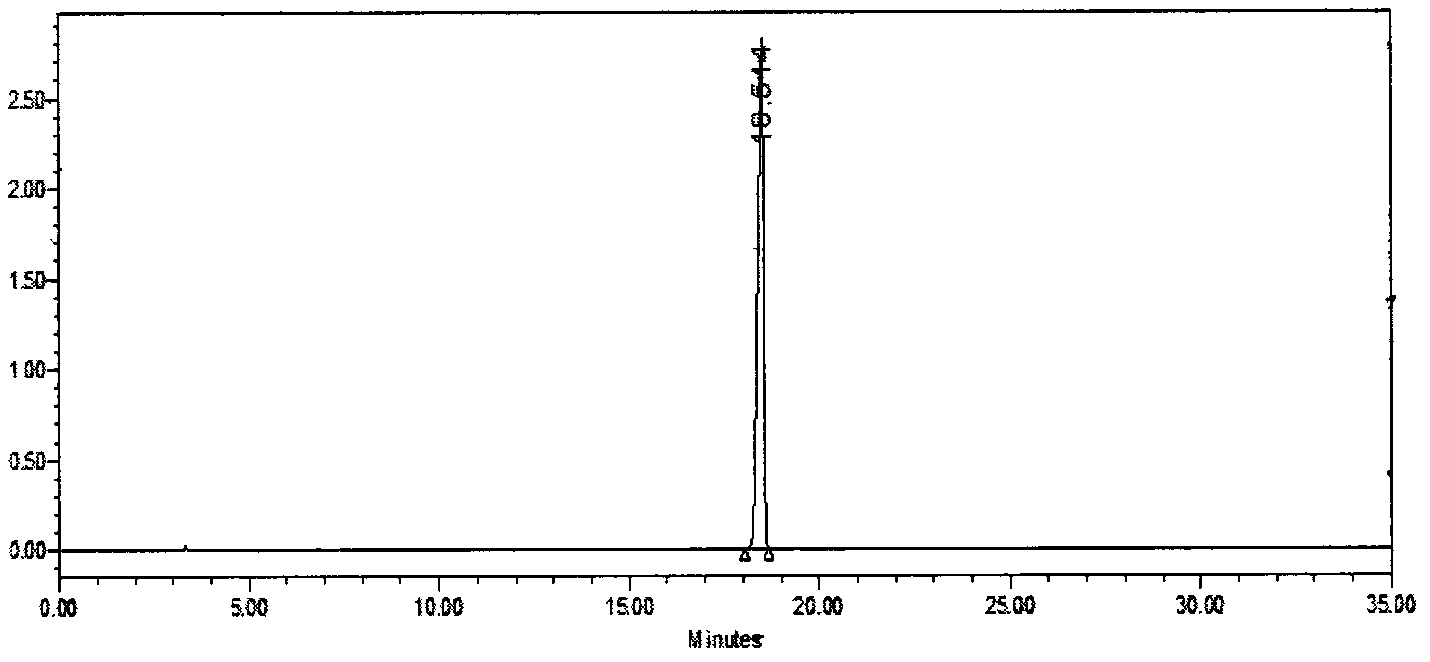
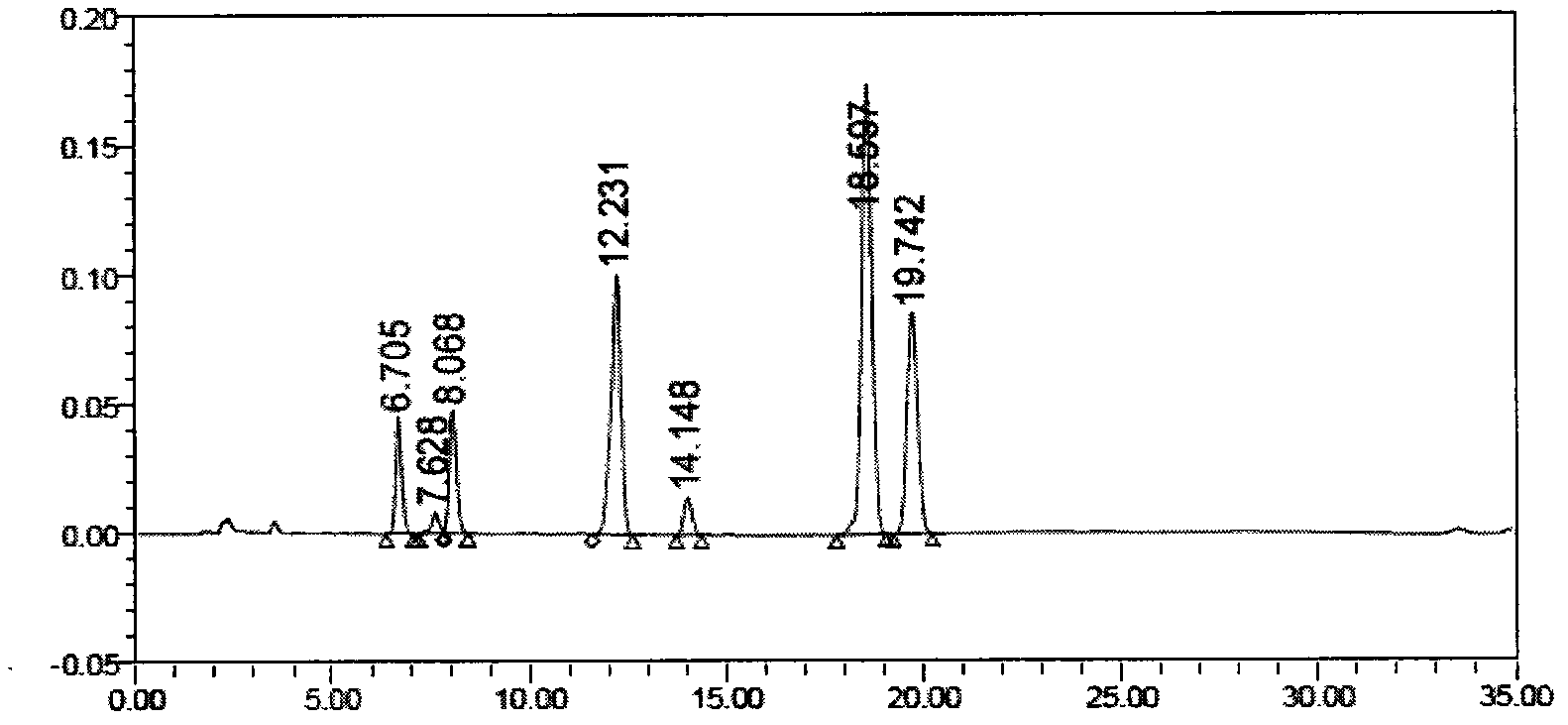
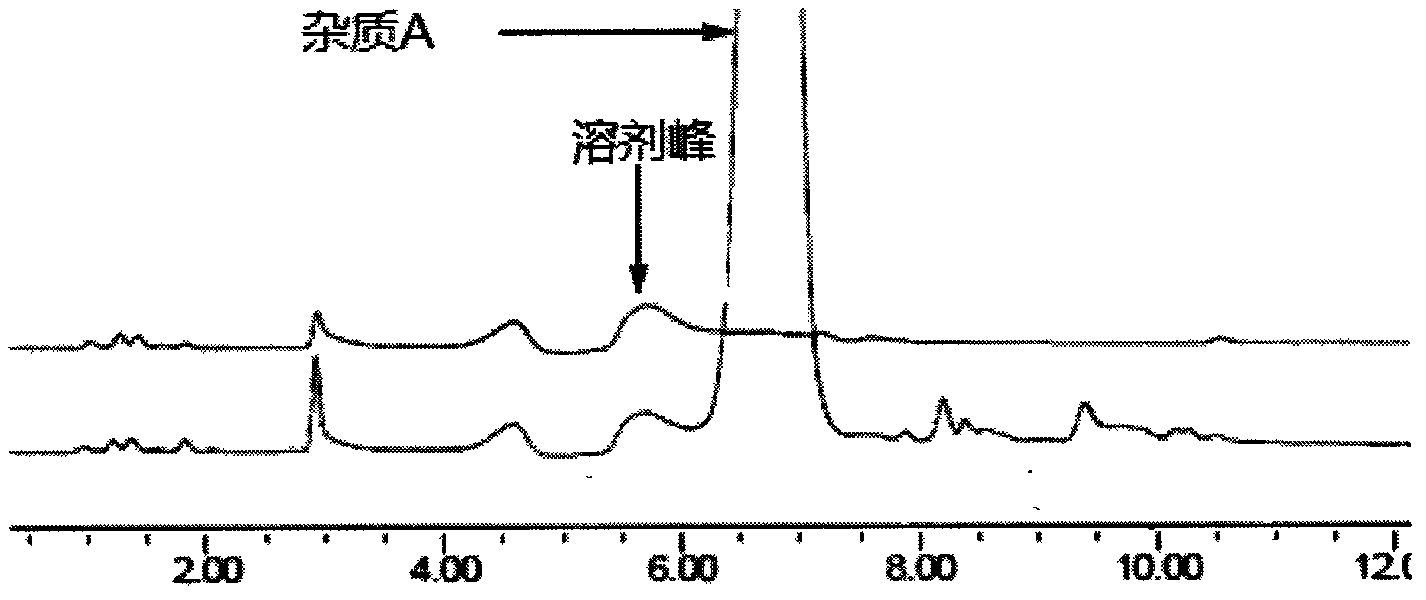
Method for separating and measuring tebipenem pivoxil related substances
The invention provides a method for separating and measuring tebipenem pivoxil related substances. According to the method, a chromatographic column adopts a C18 chromatographic column; a mobile phase is a mixed solution consisting of acetonitrile and a phosphate solution with the pH value being 3.0 to 6.0; column temperature is 38 to 40 DEG C; detection wavelength is 220 nm; flow velocity is 0.8 to 1.2 ml / min; gradient elution is performed by changing the ratio of the acetonitrile to the phosphate solution in the mobile phase. The method can effectively separate and measure six impurities of tebipenem pivoxil, has good separation degree and good peak shape, and can be applied to quality control of tebipenem pivoxil raw material medicines and tebipenem pivoxil medicine preparations.
Owner:深圳万乐药业有限公司
Preparation method for tebipenem pivoxil
Owner:SHANGHAI INST OF PHARMA IND
A kind of preparation method of tipipenem ester
The invention relates to the technical field of medicines and particularly relates to a method for preparing Tebipenem pivoxil. The method for preparing the Tebipenem pivoxil, provided by the invention, at least comprises the following steps: (1) condensation reaction; (2) hydrogenation reaction; (3) esterification reaction; and (4) purification and refining.
Owner:科兴生物制药股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com