Novel synthetic method of tebipenem pivoxil side chain
A technology of tipipenem and a synthesis method, applied in the field of medicinal chemistry, can solve the problems of being unsuitable for industrialized production, having no reaction conditions, and high raw material prices, and achieving the advantages of convenient industrialized production, high operational safety, and cheap and easy-to-obtain raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
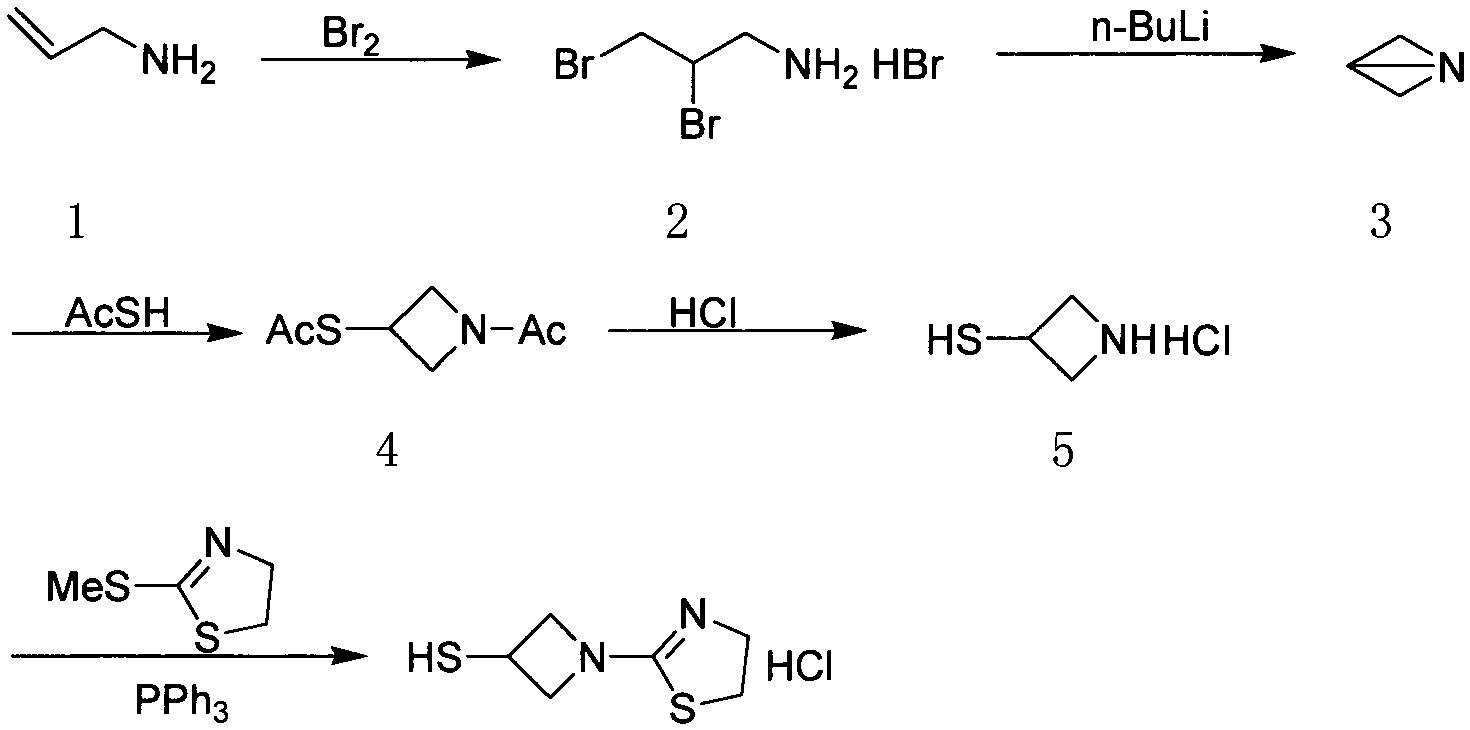
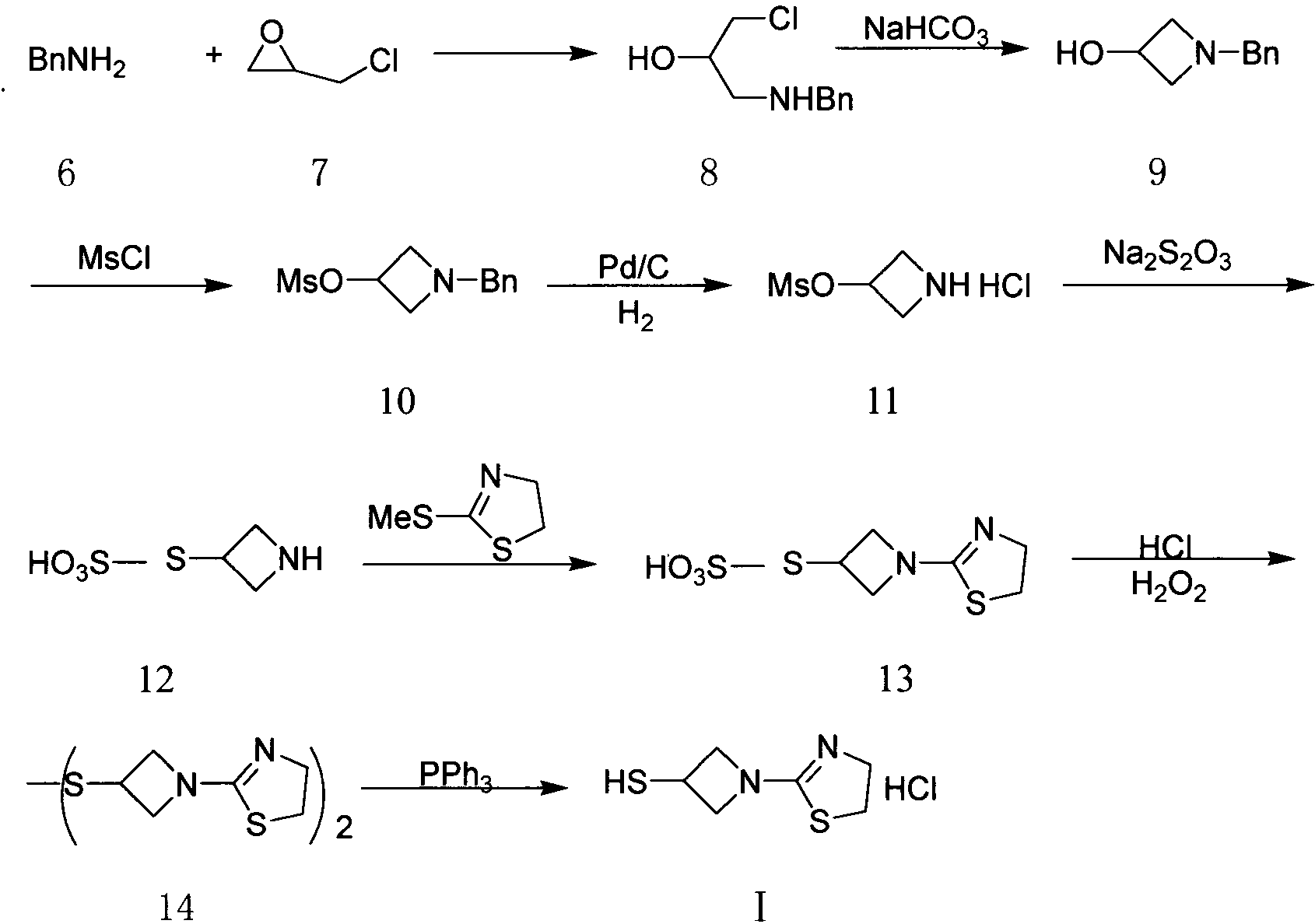
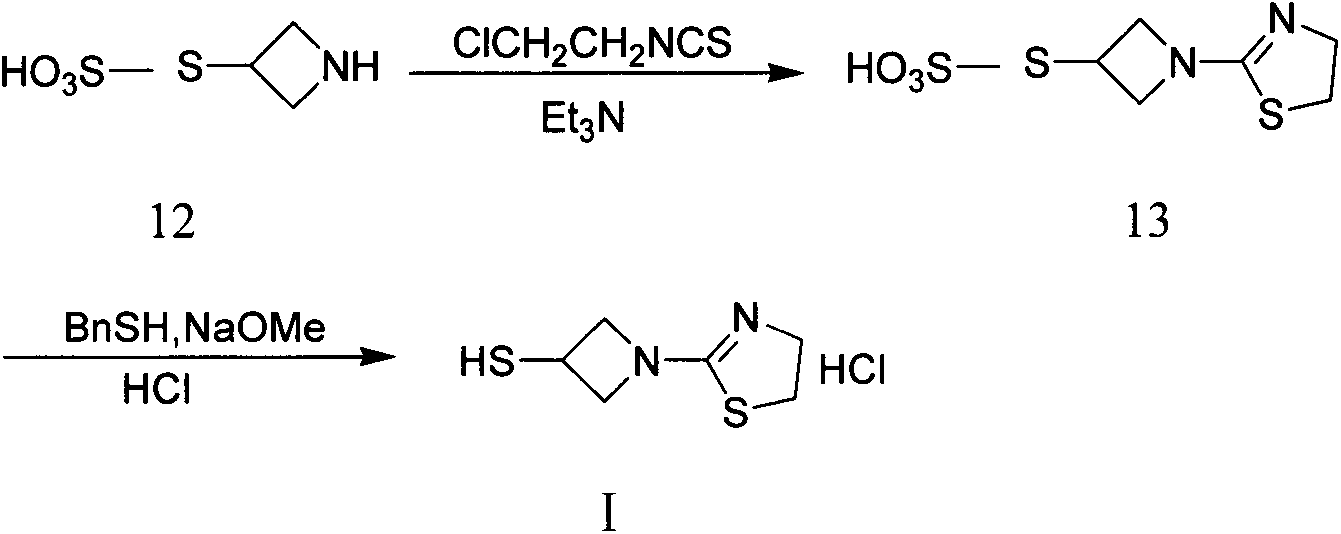
[0028] In order to make the technical means, creative features, work flow, and use methods of the present invention achieve the purpose and effect easy to understand, the present invention will be further elaborated below in conjunction with specific reaction formulas.
[0029]
[0030] Synthesis of compound II:
[0031] Add 1200 milliliters of ethanol and 111.3 grams of benzaldehyde (1.05mol) in the reaction flask, under stirring, add 110 milliliters of 25% ammoniacal liquor (1.60mol), after stirring for 15 minutes, add dropwise 92 grams of epichlorohydrin (1.00mol), control The temperature is lower than 40°C, the dropwise addition is completed, react at 35-40°C for 8 hours, and then react at room temperature for 15 hours. After the gas phase detection of epichlorohydrin almost disappears completely, the reaction solution is concentrated to remove about 1 / 3 of the volume of ethanol, and then Add 800 milliliters of toluene, under stirring, control the temperature at 35-40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com