Preparation method of tebipenem pivoxil and intermediate thereof
A technology for tipipenem ester and intermediates, which is applied in the field of preparation of tipipenem ester, can solve the problems of incomplete reaction, complicated operation, low purity, etc., achieve reduced solvent usage, ensure washing effect, and mild reaction conditional effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
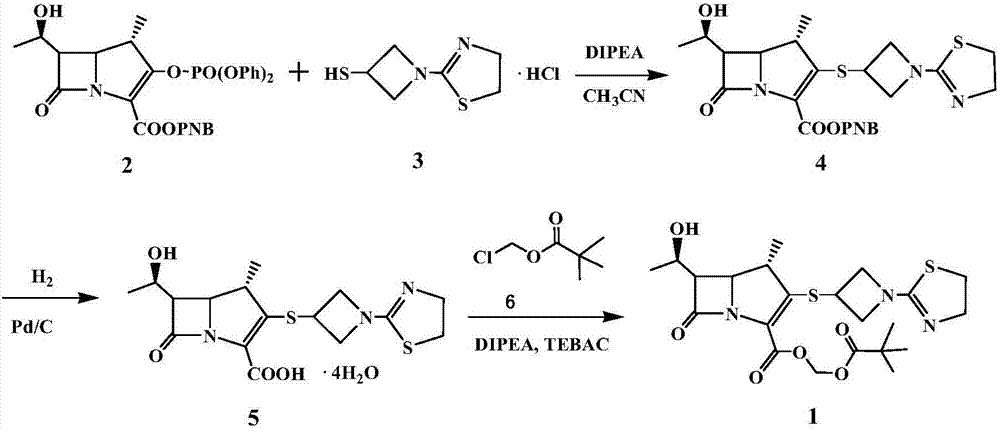
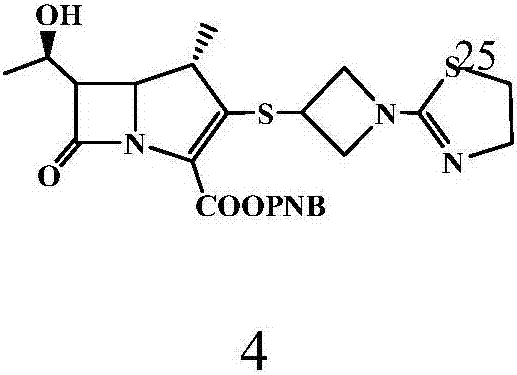
[0063] 4-Nitrobenzyl (1R, 5S, 6S)-6-[(R)-1-hydroxyethyl]-1-methyl-2-[1-(1,3-thiazolin-2-yl) Preparation of azetidin-3-yl]thio-1-carbapenim-2-ene-3-carboxylate (compound 4)
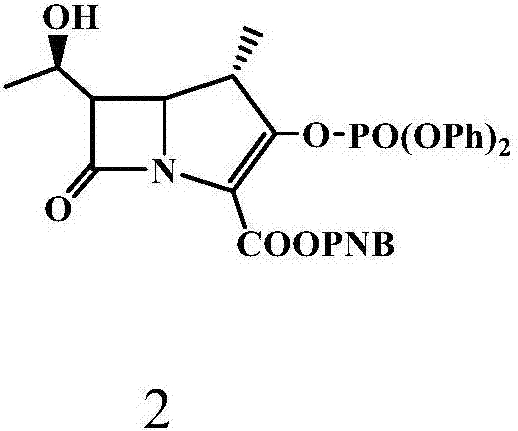
[0064] Under nitrogen protection, 2.5 L of acetonitrile was added to a 5 L reaction flask, cooled to -4-5°C, 256.5 g (0.432 mol) of compound 2 (MAP) and 100 g (0.475 mol) of compound 3 (TAT) were added, and stirred evenly. Slowly add 56.0 g (0.433 mol) of N,N-diisopropylethylamine (DIPEA) dropwise at -4~5°C, and the dropping time is controlled within 1~2h. After dropping, keep warm at -4~5°C for 3 hours, TLC (thin layer chromatography, developing solvent is methanol: ethyl acetate = 1:4) monitor the reaction until the reaction is complete, add 1.25L of water to the four-necked bottle, and heat up To 0 ~ 5 ℃, heat preservation and stirring for 0.5h. After heat preservation, filter with suction to obtain 252g of solid matter, beat with 1008g of absolute ethanol at 20-25°C for 30 minutes, filter, and vacuum...
Embodiment 2
[0066] 4-Nitrobenzyl (1R, 5S, 6S)-6-[(R)-1-hydroxyethyl]-1-methyl-2-[1-(1,3-thiazolin-2-yl) Preparation of azetidin-3-yl]thio-1-carbapenim-2-ene-3-carboxylate (compound 4)
[0067] Under nitrogen protection, 2.5 L of acetonitrile was added to a 10 L reaction flask, the temperature was lowered to -4~5°C, 256.5 g (0.432 mol) of compound 2 (MAP) and 100 g (0.475 mol) of compound 3 (TAT) were added, and stirred evenly. Slowly add DIPEA56.0g (0.433mol) dropwise at -4~5°C, and the dropping time is controlled within 1~2h. After dropping, keep warm at -4~5°C for 3 hours, monitor the reaction with TLC (developing agent: methanol:ethyl acetate=1:4) until the reaction is complete, add 2.5L of water into the four-necked bottle, heat up to 0~5°C, Keep stirring for 0.5-1h. Heat preservation is completed, suction filtration, solid 251g, with 20~25 ℃ absolute ethanol 1500g, beat for 45 minutes, filter, filter cake vacuum-dried to obtain solid 220.2g, yield 98.4%, purity 99.6%.
Embodiment 3
[0069] 4-Nitrobenzyl (1R, 5S, 6S)-6-[(R)-1-hydroxyethyl]-1-methyl-2-[1-(1,3-thiazolin-2-yl) Preparation of azetidin-3-yl]thio-1-carbapenim-2-ene-3-carboxylate (compound 4)
[0070] Under nitrogen protection, 2.5 L of acetonitrile was added to a 10 L reaction flask, the temperature was lowered to -4~5°C, 256.5 g (0.432 mol) of compound 2 (MAP) and 100 g (0.475 mol) of compound 3 (TAT) were added, and stirred evenly. Slowly add DIPEA56.0g (0.433mol) dropwise at -4~5°C, and the dropping time is controlled within 1~2h. After dropping, keep warm at -4~5°C for 3 hours, monitor the reaction with TLC (methanol:ethyl acetate=1:4) until the reaction is complete, add 5L of water into the four-necked bottle, raise the temperature to 0~5°C, keep stirring for 0.5 ~1h. After heat preservation, suction filtration, 250.6g of solid, 2506g of absolute ethanol at 20-25°C, beating for 60 minutes, filtration, and vacuum drying of the filter cake gave 220.4g of solid, with a yield of 98.5% and a p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com