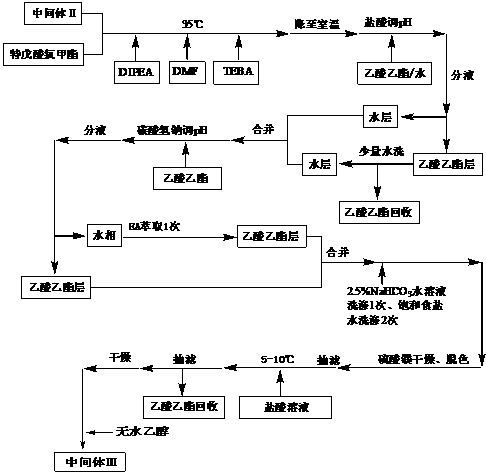
The preparation method of tipipenem ester
A technology of tipipenem ester and volume ratio, which is applied in the field of preparation of tipipenem ester, can solve problems such as unfavorable product performance, and achieve the effects of precise control of raw materials and processes, efficient synthesis process, and stable market supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
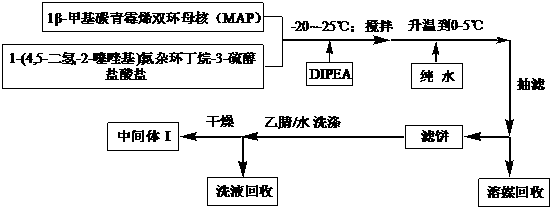
[0060] Preparation of Intermediate I:
[0061] Weigh 252.9 g (1.2 mol) of 1-(4,5-dihydro-2-thiazolyl) azetidine-3-thiol hydrochloride and 594.5 g of 1β-methylcarbapenem bicyclic nucleus (1mol) was added to 1189ml of diisopropylethylamine, stirred and reacted at -25°C for 4 hours, after the reaction was completed, 600ml of pure water was added, the temperature was raised to 2°C within 30 minutes, suction filtered, and the filter cake was washed with acetonitrile water (V acetonitrile: V water = 2:3) 297ml of the solution was washed and dried to obtain 457.4g of intermediate I with a calculated yield of 88.3% and a measured purity of 99.0%.
Embodiment 2
[0063] Preparation of Intermediate I:
[0064] Weigh 274.0 g (1.3 mol) of 1-(4,5-dihydro-2-thiazolyl) azetidine-3-thiol hydrochloride and 594.5 g of 1β-methyl carbapenem bicyclic nucleus (1mol) was added to 1783ml of diisopropylethylamine, stirred and reacted at -20°C for 3.5 hours, after the reaction was completed, 900ml of pure water was added, the temperature was raised to 0°C within 30 minutes, suction filtered, and the filter cake was washed with acetonitrile water (V acetonitrile: V water = 2:3) 297ml of the solution was washed and dried to obtain 458.4g of intermediate I with a calculated yield of 88.5% and a measured purity of 99.2%.
Embodiment 3
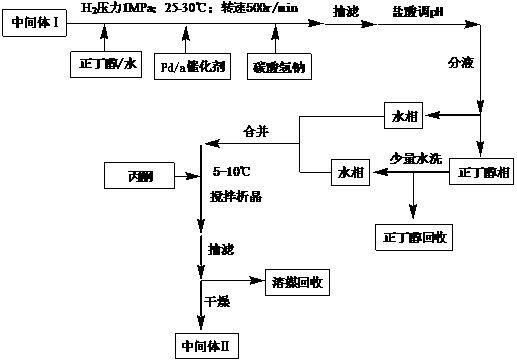
[0066] Preparation of Intermediate II:
[0067] The 228.7g intermediate I obtained in Example 1 was mixed with the volume ratio of n-butanol aqueous solution (the volume ratio of n-butanol and water is 3:1) 1150ml, palladium carbon catalyst (0.5% palladium) 18.3g and sodium bicarbonate 274.5g Mix and react for 4 hours under the conditions of hydrogen pressure 1MPa, 25~30°C, and rotation speed 500r / min. After the reaction, filter with suction, adjust the pH of the filtrate to 5.5 with 4mol / l hydrochloric acid, and separate the liquids to obtain the aqueous phase and n-butanol Phase; lower the temperature to 5~6°C, slowly add acetone 6 times its volume while stirring the water phase, crystallize the solution, filter with suction, and dry to obtain 150.3g of intermediate II, the calculated yield is 88.9%, and the purity is determined 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com