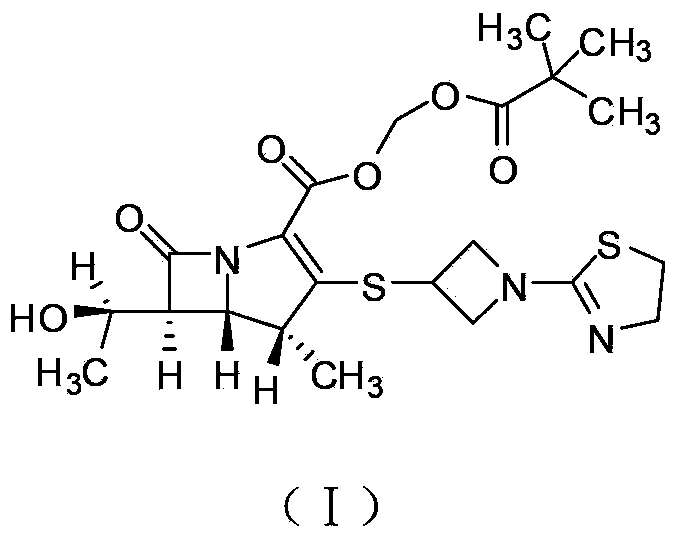
Tebipenem pivoxil industrial preparation method
A technology of tipipenem ester and tipipenem, applied in the field of medicinal chemistry, can solve the problems of instability of iodomethyl pivalate, unsuitable for industrialized production, complicated post-processing operations and the like, and achieves simple and easy post-processing operations. , the use of less solvent, the effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
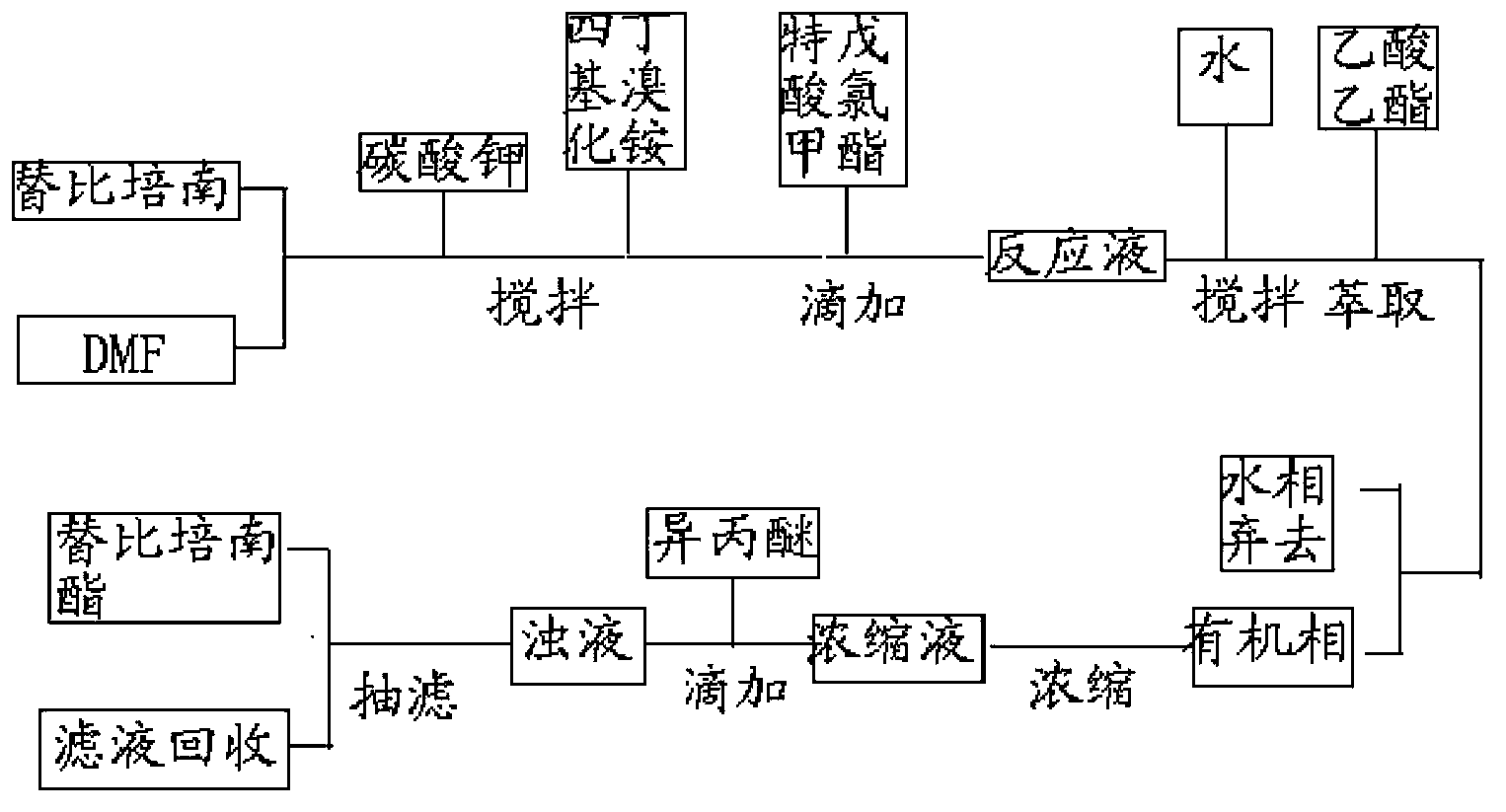
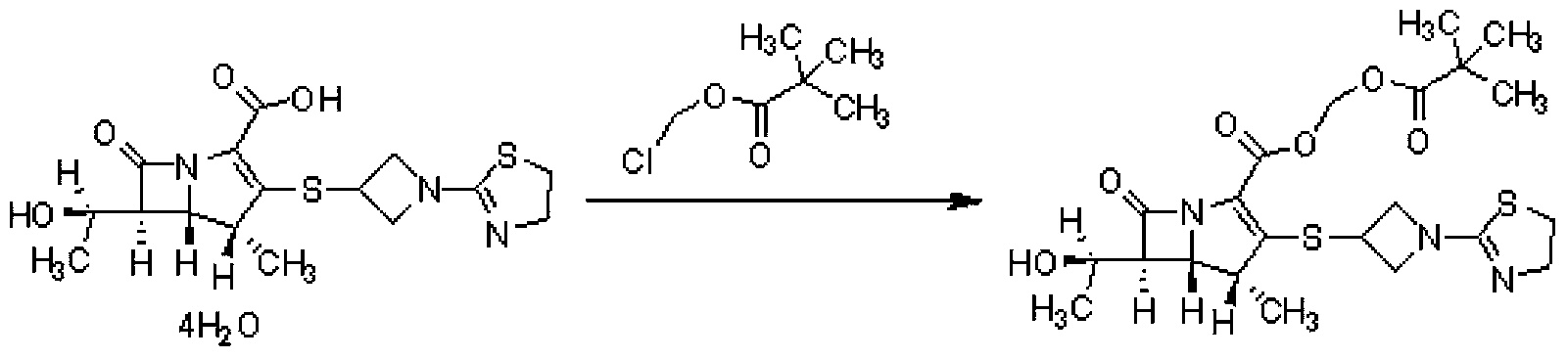
[0029] 18g of tipipenem, 162mL of N,N-dimethylformamide, 6.54g of potassium carbonate, and 0.45g of tetrabutylammonium bromide were reacted at 25°C for 60min, and chloroform pivalate was added dropwise at this temperature 8.93g ester, after the reaction is completed, add 320ml of water and stir for 10 minutes, add 160ml of ethyl acetate to extract, extract the water phase with 160ml of ethyl acetate once, combine ethyl acetate, wash with water, separate phases, wash the ethyl acetate phase with 640ml of water, Add 60 g of anhydrous sodium sulfate to the ethyl acetate phase, dry and decolorize with activated carbon, concentrate the filtrate, add 162 mL of isopropyl ether dropwise at 25°C, stir and crystallize, filter, and dry to obtain 17.64 g of a white solid. The HPLC detection purity was 99.87%, and the yield was 89.7%.
[0030] TBPN 1 H NMR data (CDCl 3 ): 5.989-5.978ppm (1H, d, 5.5, H-13), 5.858-5.847ppm (1H, d, 5.5, H-13), 4.436-4.390ppm (2H, m, H-22e, H-24e ), 4.232-4...
Embodiment 2
[0033] React 18g of tipipenem, 162ml of N,N-dimethylformamide, 10.92g of potassium carbonate, and 0.45g of benzyltriethylammonium chloride at 20°C for 70min, and add pivalic acid dropwise at this temperature Chloromethyl ester 17.8g, after the reaction is completed, add 320ml of water and stir for 10 minutes, add 160ml of ethyl acetate for extraction, extract the water phase with 160ml of ethyl acetate once, combine ethyl acetate, wash with water, separate phases, wash the ethyl acetate phase with 640ml of water, Add 60 g of anhydrous sodium sulfate to the ethyl acetate phase, dry and decolorize the activated carbon, concentrate the filtrate to a certain volume, add 180 mL of methyl tert-butyl ether dropwise at 20°C, stir for crystallization, filter, and dry to obtain 17.30 g of a white solid. The HPLC detection purity was 99.75%, and the yield was 88%.
Embodiment 3
[0035] React 18g of tipipenem, 162ml of N,N-dimethylformamide, 4.19g of sodium carbonate, and 0.18g of tetrabutylammonium bromide at 30°C for 50min, and add pivalate chloromethyl pivalate dropwise at this temperature 8.93g ester, after the reaction was completed, add 320ml of water and stir for 10 minutes, add 160ml of ethyl acetate to extract, extract the water phase with 160ml of ethyl acetate once, combine ethyl acetate, wash with water, separate phases, wash the ethyl acetate phase with 640ml of water, wash with ethyl acetate 60 g of anhydrous sodium sulfate was added to the ester phase, and activated carbon was dried for decolorization. The filtrate was concentrated to a certain volume, and 144 mL of n-heptane was added dropwise at 25° C., stirred for crystallization, filtered, and dried to obtain 17.36 g of a white solid. The HPLC detection purity was 99.77%, and the yield was 88.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com