Preparation method of tebipenem pivoxil
A technology of tipipenem and pivoxil, which is applied in the field of preparation of tipipenem pivoxil, can solve the problem that post-processing is not reasonable enough, the yield of tipipenem pivoxil and intermediate products declines, and the effect of Problems such as large-scale production of penem pivoxil to achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
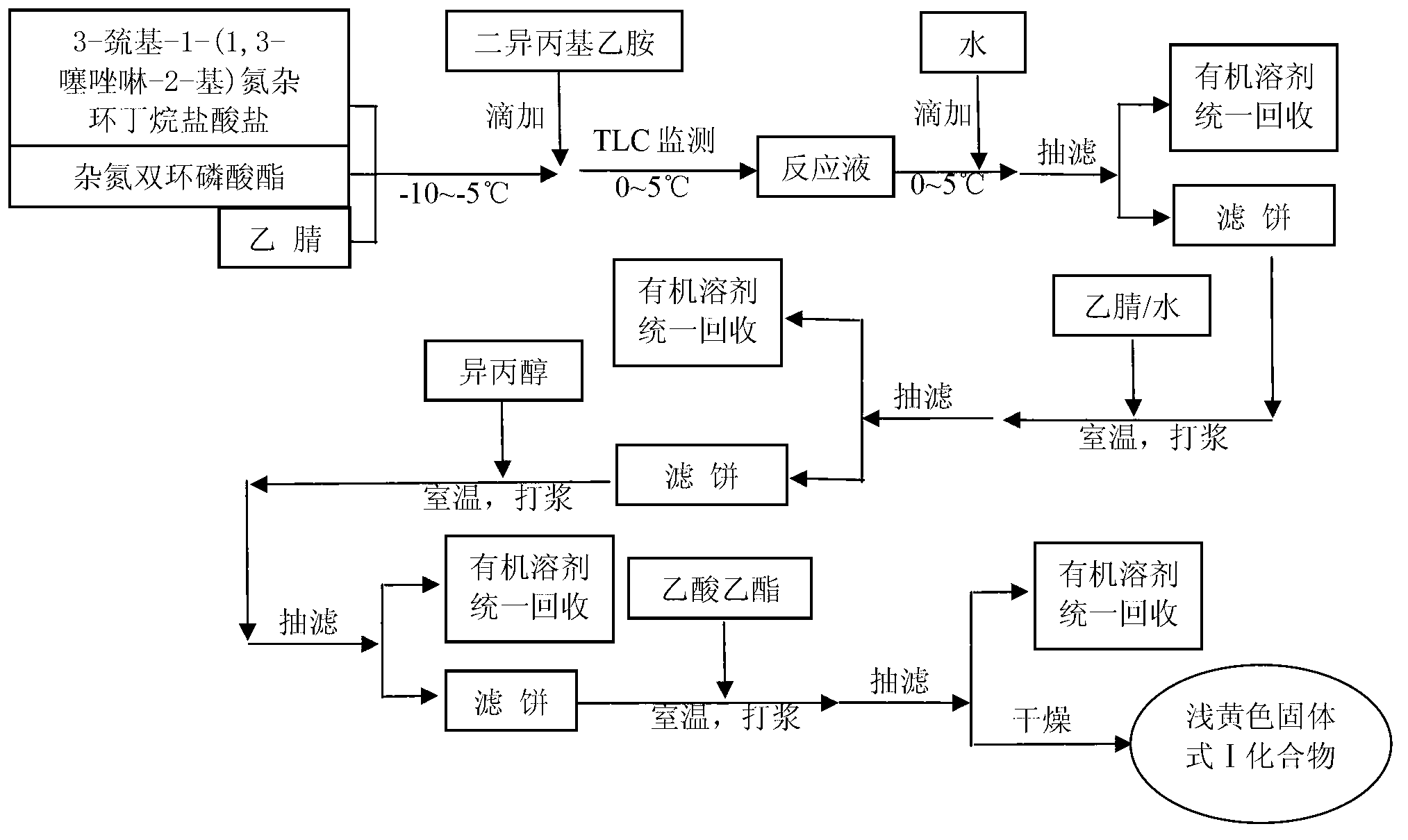
[0055] Embodiment 1: the preparation of formula I compound
[0056] In a 50L reactor, add 2750g (4.626mol) of azabicyclic phosphate, 1072g (5.088mol) of 3-mercapto-1-(1,3-thiazolin-2-yl)azetidine hydrochloride, 18.33 Stir with kg acetonitrile, cool down to -10~-5°C, within 1 hour, add 1434g (11.102mol) diisopropylethylamine dropwise, after the drop is complete, keep stirring at 0~5°C for 5~6 hours, then 0~5°C , add 12.0kg of water dropwise within 1 hour, stir for 30 minutes, filter with suction, add the filter cake to the acetonitrile aqueous solution composed of 2.5kg of water and 2.4kg of acetonitrile, stir at room temperature for 1 hour, filter with suction, add the filter cake to 3.9kg of isopropyl In alcohol, stir at room temperature (18-30°C) for 12-17 hours, filter with suction, add the filter cake to 0.9kg ethyl acetate, stir at room temperature (18-30°C) for 1 hour, filter with suction, and dry the filter cake in vacuum at 35°C After about 4 hours, 1558 g of light ye...
Embodiment 2
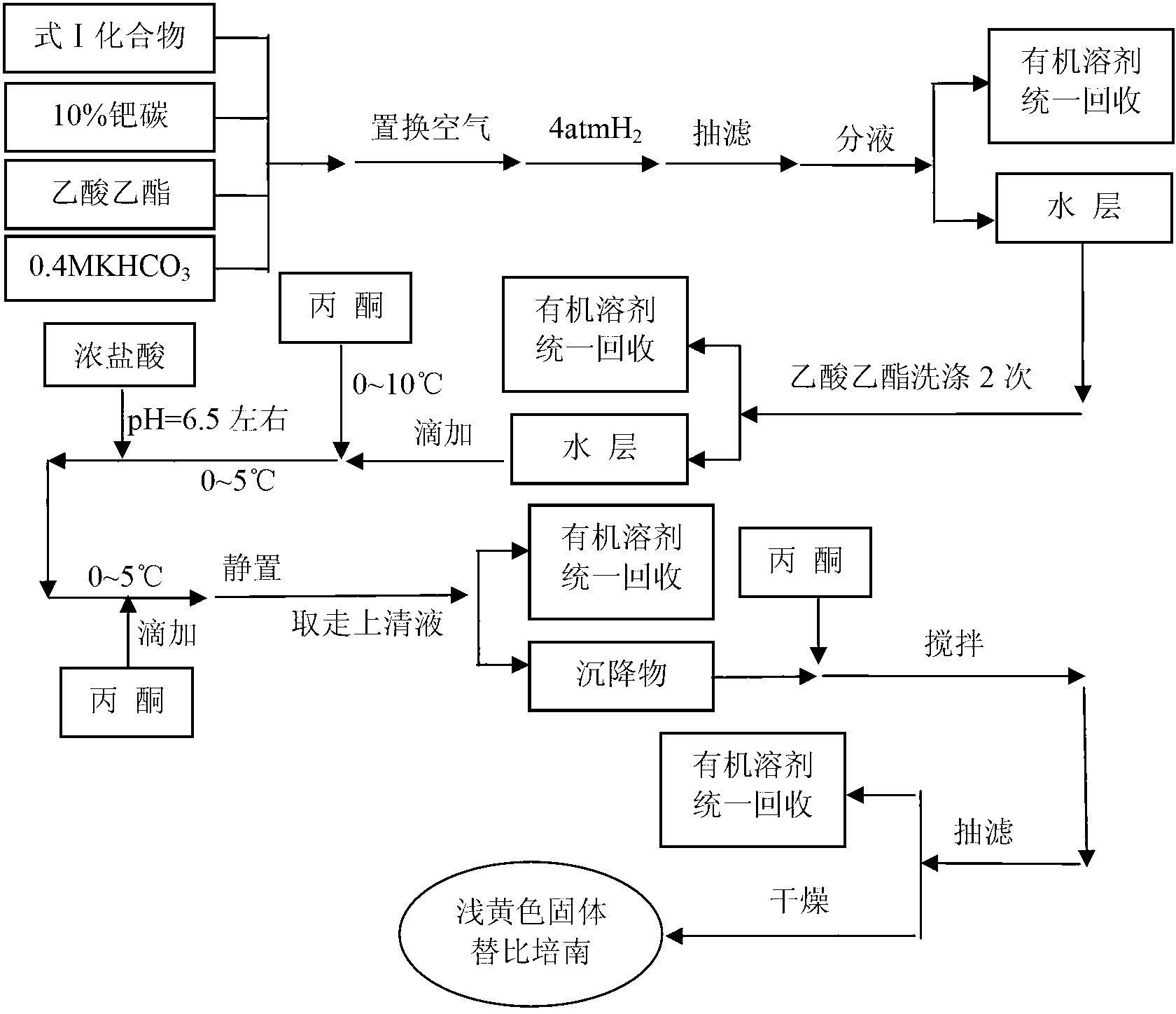
[0057] Example 2: Preparation of tipipenem
[0058] In a 50L hydrogenation kettle, add 1550g (2.989mol) of the compound of formula I, 775g of 10% palladium carbon, 17.1kg of ethyl acetate, 7.5kg of 0.4M potassium bicarbonate, replace the air, and pass hydrogen at 4 atmospheres for 12 to 16 hours (to the reaction system no longer absorb hydrogen); filter with suction, wash the filter cake with 0.9kg ethyl acetate, let stand to separate the layers, and wash the water layer with ethyl acetate 3 times (4.5kg each time);
[0059] Add the above washed water layer dropwise to 20kg of acetone at 0~10°C within 1 hour, stir for 30 minutes, then adjust the pH value to about 6.5 with concentrated hydrochloric acid at 0~5°C, then add 16kg of acetone dropwise within 30 minutes Acetone, after dripping, stir for 30 minutes, let stand for 10-15 hours, remove the supernatant and add 11.8kg of acetone to the sediment, stir vigorously for 2 hours, filter with suction, wash the filter cake with 1....
Embodiment 3
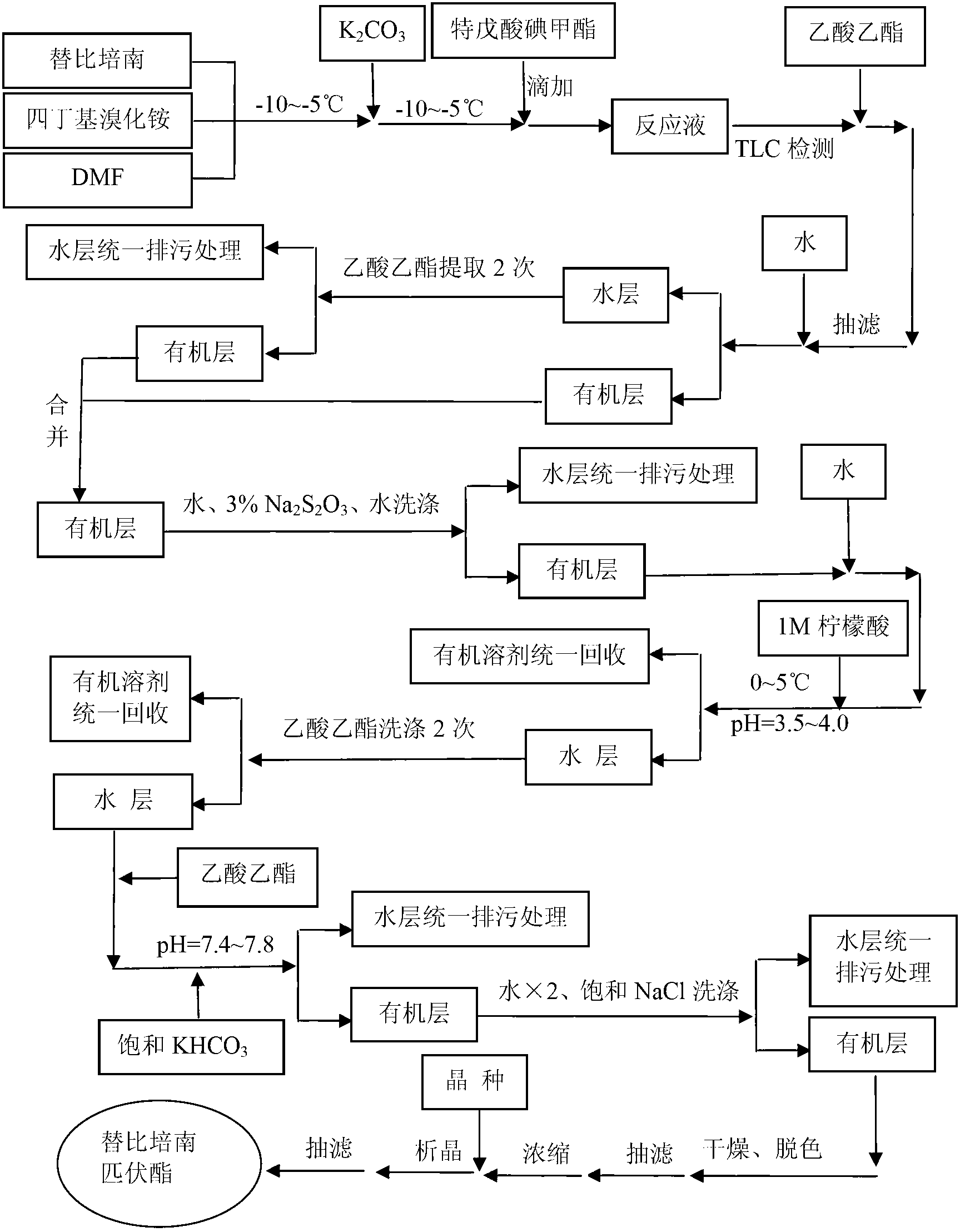
[0060] Embodiment 3: Preparation of tipipenem pivoxil
[0061] In a 20L four-neck flask, add 725g (1.890mol) tipipenem, 16.7g (51.8mmol) tetrabutylammonium bromide, 6.9kg anhydrous DMF and stir in turn, and add 313g (2.265mol) ) anhydrous potassium carbonate, stirred for 30 minutes; -10~-5℃, added dropwise 549g (2.268mol) iodomethyl pivalate within 45 minutes, after dropping, stirred at -10~-5℃ for 1~1.5 hours (TLC Monitor the reaction in time, chloroform / methanol: 5 / 1);
[0062] After completion of the reaction, the reaction solution was added to 7.7kg of ethyl acetate, suction filtered, and 25kg of water was added to the filtrate, stirred for 30 minutes, left to stand and layered into the first water layer and the first organic layer, and the first water layer was washed with ethyl acetate. The ester was extracted twice (9 kg each time) to obtain the second organic layer, and the first and second organic layers were combined and washed with 15 kg of water, 15 kg of 3% sodiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com