Oral solid preparation of tipipenem axetil and preparation method thereof
A technology for tipipenem ester and solid preparation, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., to achieve the effects of ensuring stability, ensuring stability and reducing risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
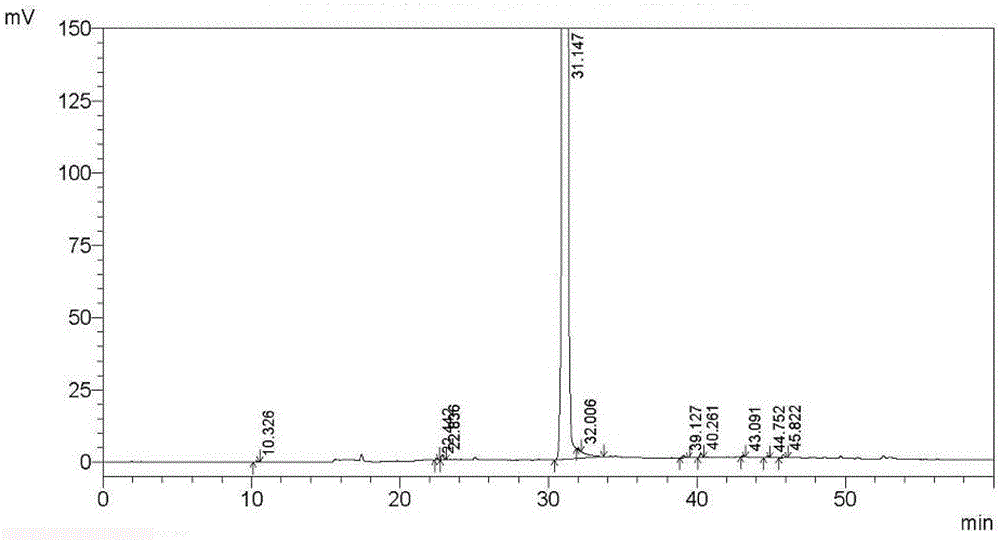
Embodiment 1
[0092] Tipipenem axetil granules
[0093]
[0094]
[0095] 1. Preparation before ingredients
[0096] 1.1 Material and equipment verification
[0097] To ensure that the materials are accurate and available, the equipment operates normally, and some parameters are set according to the instructions.
[0098] 1.2 Humidity Control
[0099] Detect ambient humidity and fluidized bed inlet humidity requirements are 30±10%
[0100] 2 Dosing
[0101] 2.1 Configuration of the adhesive
[0102] Weigh ethyl cellulose according to the adhesive formula, dissolve it in absolute ethanol by stirring (or ultrasonically) until it becomes clear, and pass the solution through a 60-mesh sieve for later use.
[0103] 2.2 Configuration of barrier coating material
[0104] According to the prescription of the isolation layer, ethyl cellulose and triethyl citrate were weighed and dissolved in absolute ethanol by stirring (or ultrasonically) until they became clear, and the solution was pa...
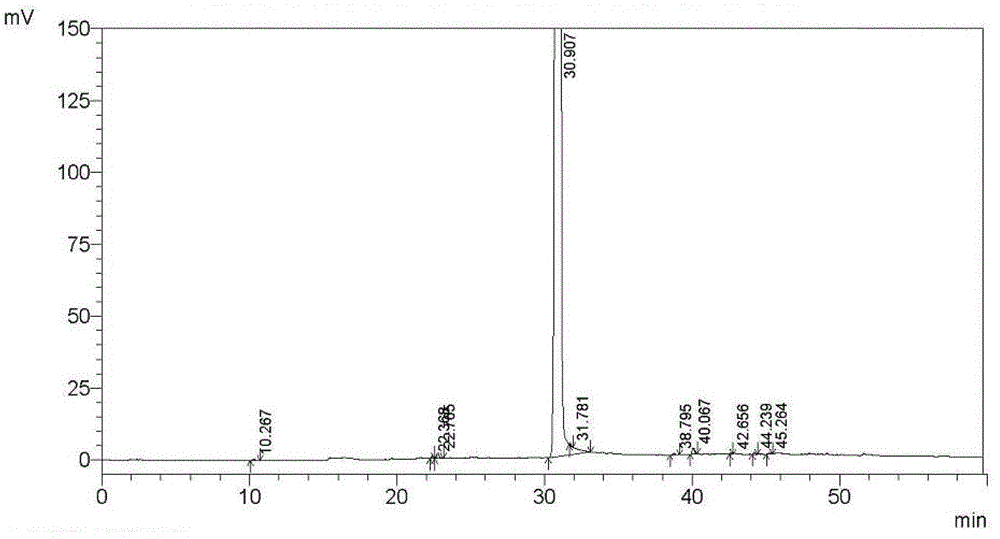
Embodiment 2
[0137] Tipipenem axetil granules
[0138]
[0139]
[0140] 1. Preparation before ingredients
[0141] 1.1 Material and equipment verification
[0142] To ensure that the materials are accurate and available, the equipment operates normally, and some parameters are set according to the instructions.
[0143] 1.2 Humidity Control
[0144] Detect ambient humidity and fluidized bed inlet humidity requirements are 30±10%
[0145] 2 Dosing
[0146] 2.1 Configuration of the adhesive
[0147] Weigh shellac according to the adhesive formula, dissolve it in absolute ethanol by stirring (or ultrasonically) until it becomes clear, and pass the solution through a 60-mesh sieve for later use.
[0148] 2.2 Configuration of barrier coating material
[0149] According to the prescription of the isolation layer, ethyl cellulose and castor oil were weighed and dissolved in absolute ethanol by stirring (or ultrasonically) until they became clear, and the solution was passed through a...
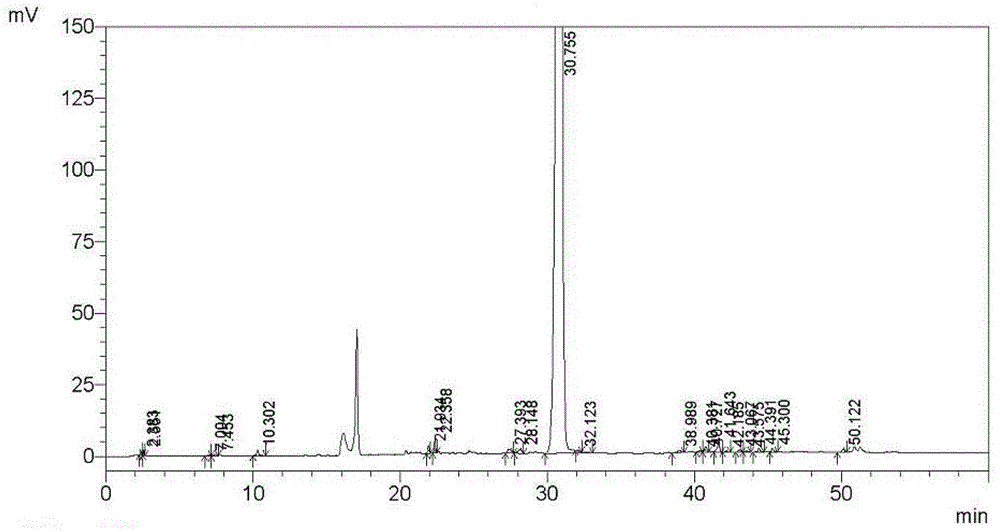
Embodiment 3
[0182] Tipipenem axetil granules
[0183]
[0184]
[0185] 1. Preparation before ingredients
[0186] 1.1 Material and equipment verification
[0187] To ensure that the materials are accurate and available, the equipment operates normally, and some parameters are set according to the instructions.
[0188] 1.2 Humidity Control
[0189] Detect ambient humidity and fluidized bed inlet humidity requirements are 30±10%
[0190] 2 Dosing
[0191] 2.1 Configuration of the adhesive
[0192] Weigh povidone according to the adhesive formula, dissolve it in purified water by stirring (or ultrasonically) until it becomes clear, and pass the solution through a 60-mesh sieve for use.
[0193] 2.2 Configuration of barrier coating material
[0194] According to the prescription of the isolation layer, zein and castor oil were weighed and dissolved in absolute ethanol by stirring (or ultrasonication) until they became clear, and the solution was passed through a 60-mesh sieve fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com