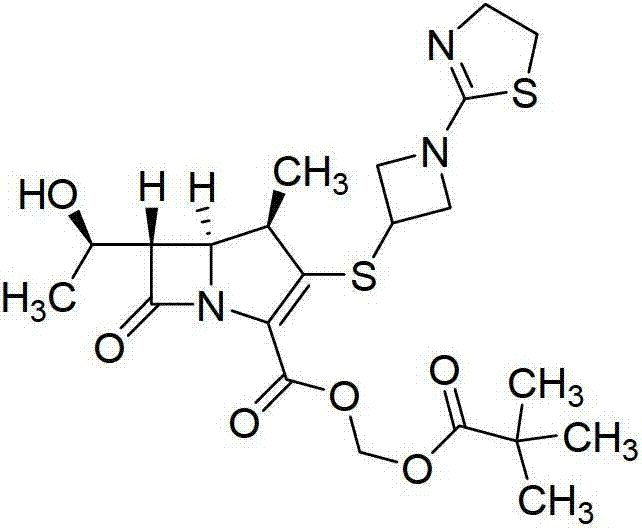
Preparation method of tebipenem pivoxil granules
A technology of tipipenem and pivoxil, which is applied in the field of preparation of tipipenem pivoxil granules, can solve the problem of rising water content, poor stability of tipipenem pivoxil, and poor dissolution of granules and other problems, to achieve the effect of low impurities, high polymer and main drug compatibility, and good dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the selection of preparation method
[0020] 1. Granulation method
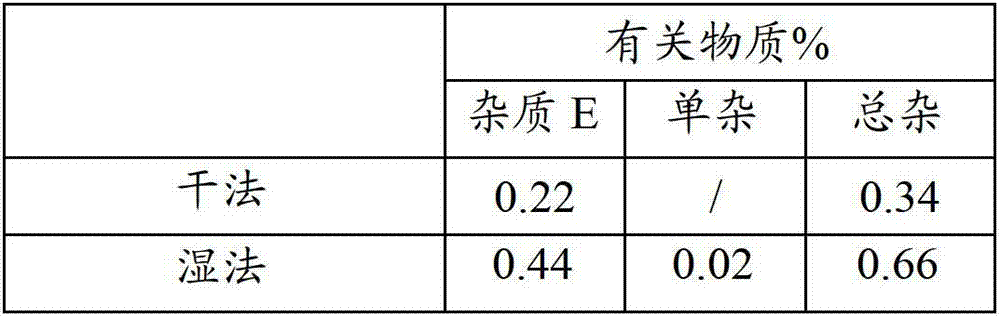
[0021] According to the prescription of tipipenem pivoxil granules of the present invention, dry granulation and wet granulation were used respectively, and then the finished product was tested for related substances (i.e. impurities). The results are shown in Table 1.
[0022] Table 1 Test results of related substances in wet granulation and dry granulation
[0023]
[0024] It can be seen from the test results that the wet granulation process increases the impurities much more than the dry granulation process, so the dry granulation process is more suitable.
[0025] 2. Selection of dry granulation conditions
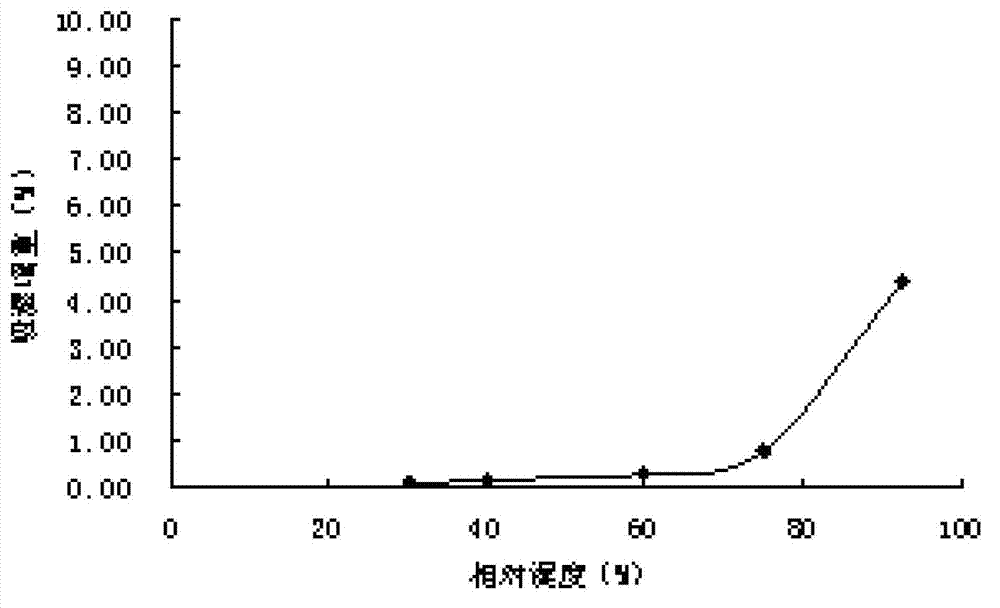
[0026] Raw material crushing particle size:
[0027] Tipipenem pivoxate is a crystalline powder. In order to mix evenly with auxiliary materials, the particle size should be kept in the same range as possible. The pulverized tipipenem pivoxate of the present invention is pa...
Embodiment 2
[0037] Embodiment 2: different proportioning tests of main and auxiliary materials
[0038] 1. Different contents of microcrystalline cellulose and sucrose
[0039] According to the prescription in Table 4, dry granulation was performed, and then the granulation condition, granule fluidity, angle of repose, granule strength, mouthfeel, and water content were used as inspection indicators for testing. The results are shown in Table 5.
[0040] Table 4 Prescriptions with different contents of microcrystalline cellulose and sucrose
[0041] Main and auxiliary materials
Prescription 1
Prescription 2
Prescription 3
Prescription 4
Prescription 5
tipipenem pivoxate
12.976g
12.976g
12.976g
12.976g
12.976g
62.50g
50.00g
37.50
25.00g
12.50g
sucrose
24.524g
37.024g
49.524g
62.024g
74.424g
1.00g
...
Embodiment 3
[0057] Embodiment 3: Preparation of tipipenem pivoxil granules of the present invention
[0058] According to the prescription of tipipenem pivoxil granule of the present invention, i.e. 32.44 parts by weight of tipipenem pivoxil, 31.25 parts by weight of microcrystalline cellulose, 183.56 parts by weight of sucrose, 2.5 parts by weight of Spartan and 0.25 parts by weight of red iron oxide (or 12.976 parts by weight of tipipenem pivoxil, 12.50 parts by weight of microcrystalline cellulose, 73.424 parts by weight of sucrose, 1.00 parts by weight of aspartame and 0.1 parts by weight red iron oxide), three batches of tipipenem pivoxil granules were prepared by dry granulation (hereinafter referred to as batch 100601, batch 100602, batch 100603), and the yields were 86.80% and 86.26% respectively , 86.60%.
[0059] Dry granulation process steps:
[0060] In an environment with a relative humidity below 60%, crush the above raw and auxiliary materials through an 80-mesh sieve, mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com