Preparation method of I type clopidogrel hydrogen sulfate
A technology for clopidogrel hydrogen sulfate and clopidogrel free base, which is applied in the field of pharmaceutical compound preparation, can solve the problems of poor reproducibility of the preparation method, reduced production cost and high production cost, and achieves that the operation method is simple and feasible, The effect of reducing production cost and high crystal purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
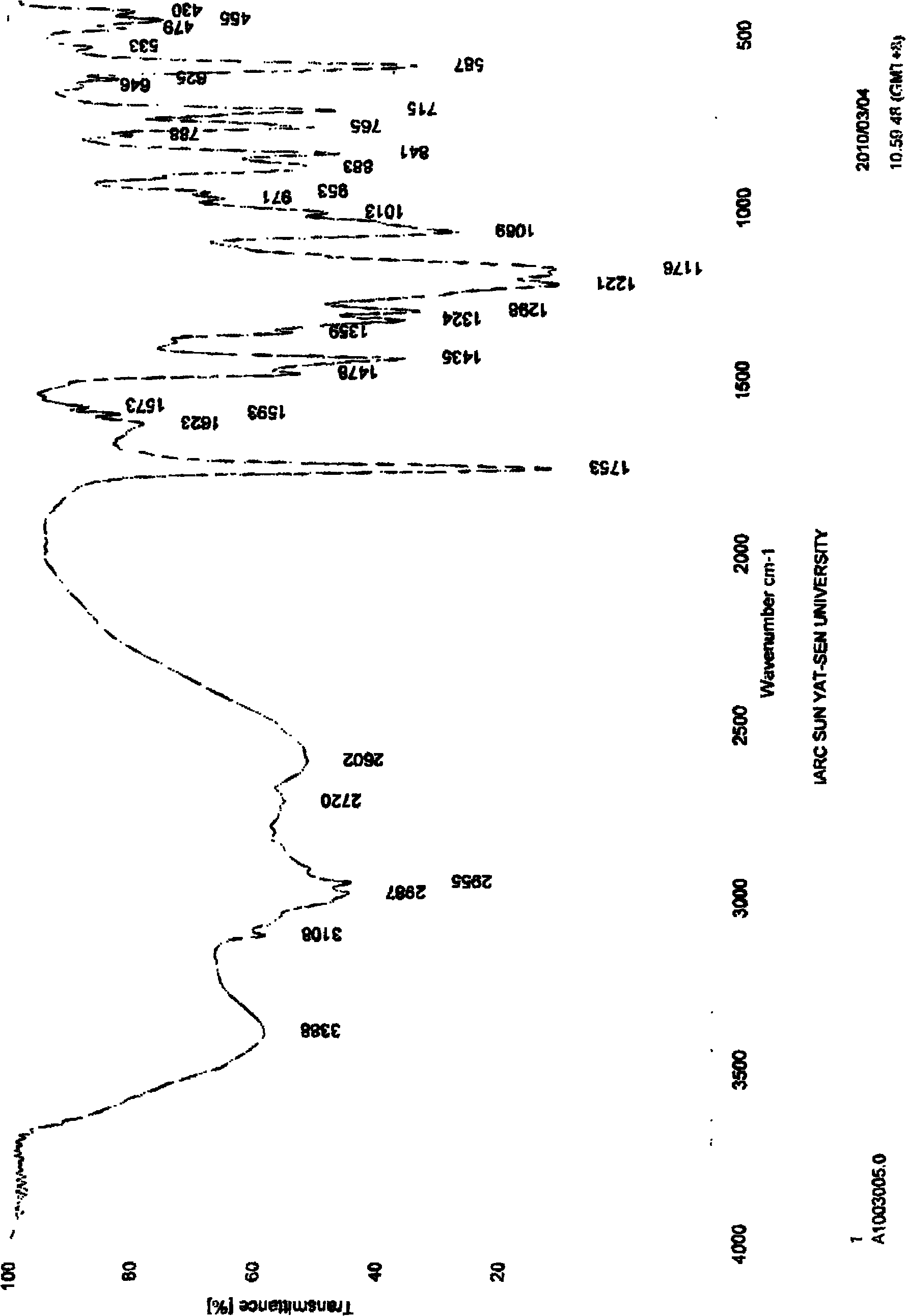
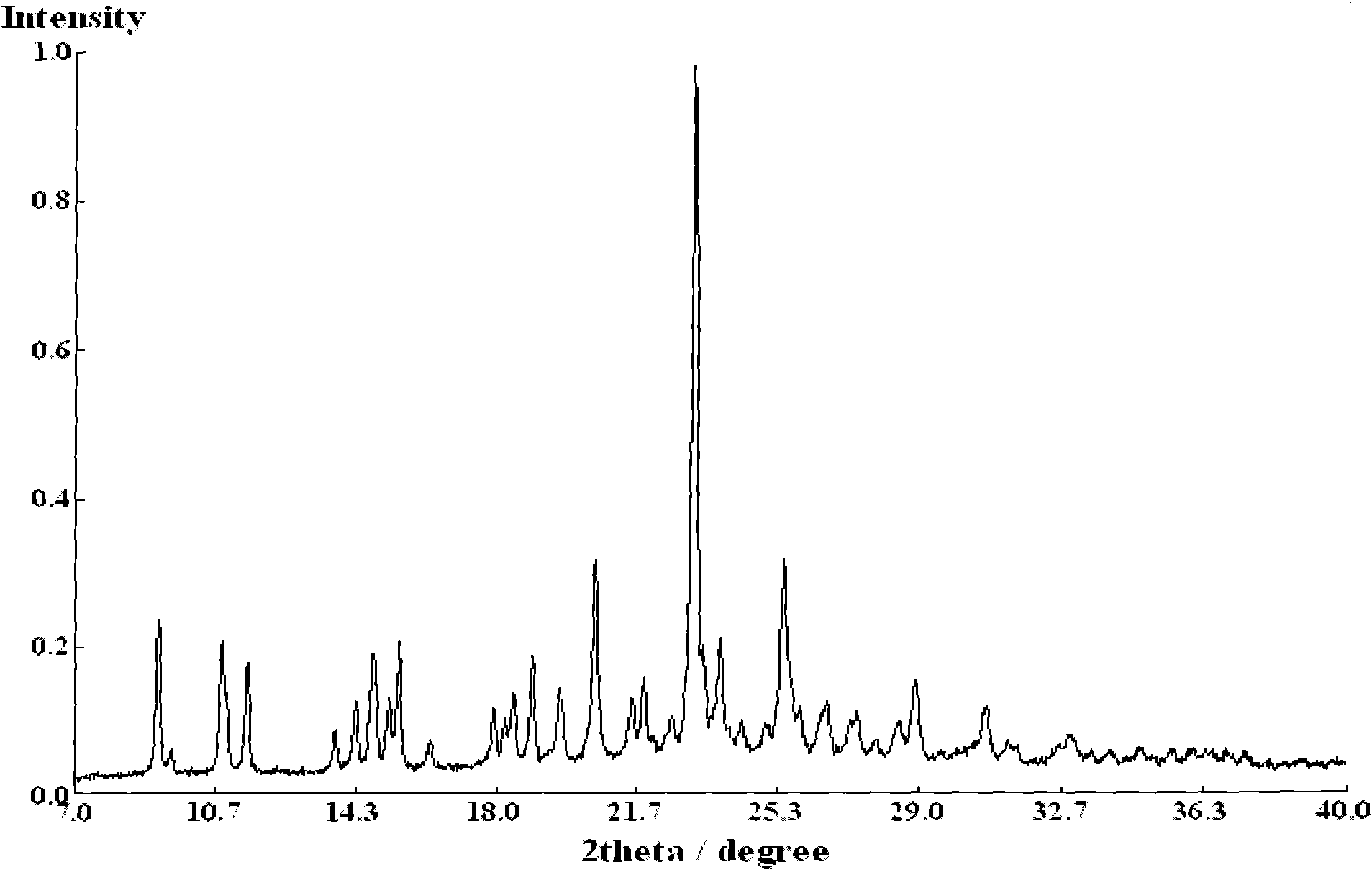
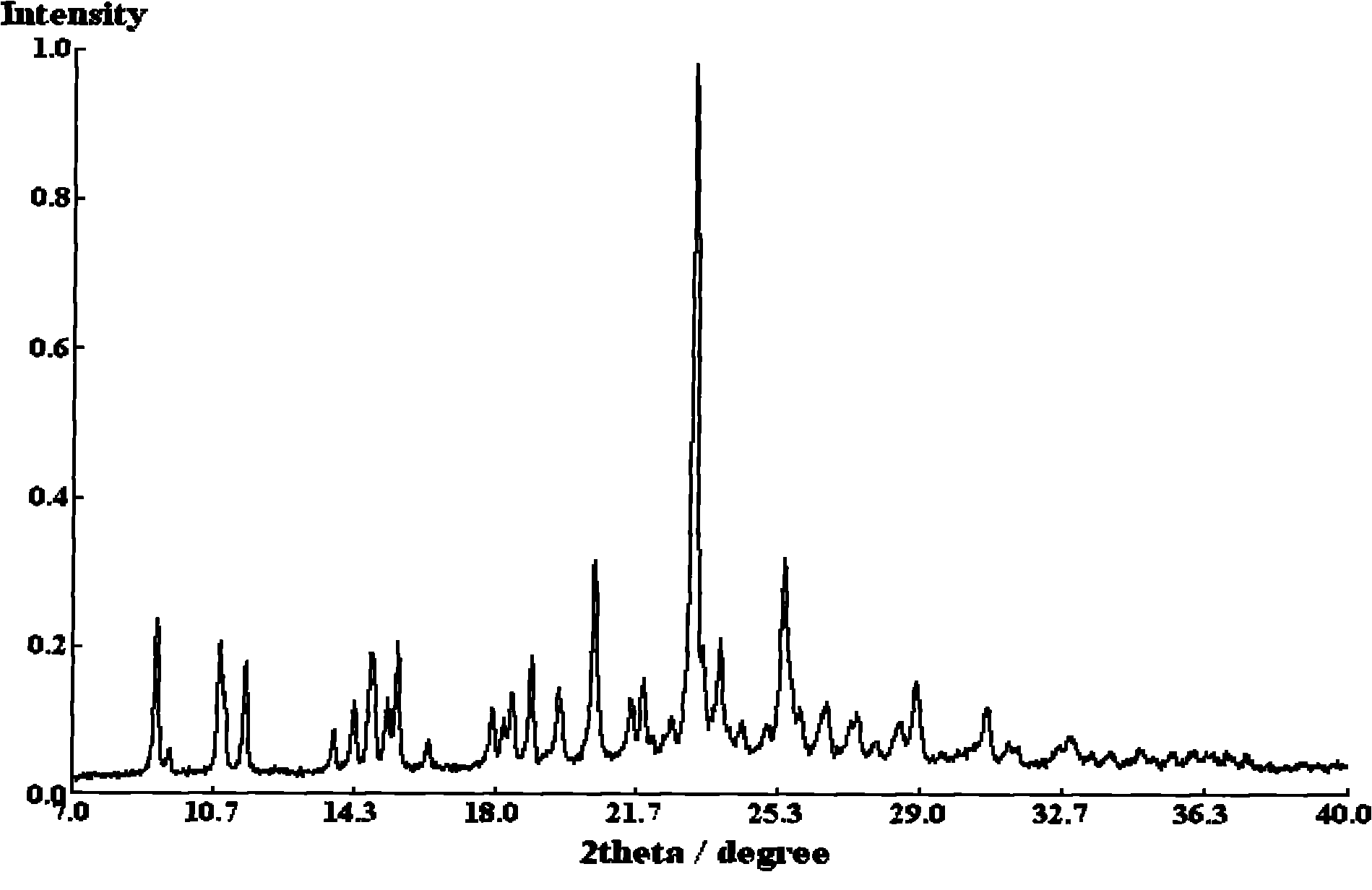
[0025] Put 0.536 g of clopidogrel free base and 5 ml of tetrahydrofuran into a 100 ml flask, stir at -14°C until the solution is clear, slowly add 0.165 g of concentrated sulfuric acid with a mass fraction of 98%, and react at -14°C for 1-2 hours , slowly raised the temperature to 20°C, and continued to stir and crystallize at this temperature for 10-12 hours. After the crystallization was completed, filter, wash with tetrahydrofuran, and drain as much as possible. Vacuum-dry the filter cake for 3 to 4 hours to obtain 0.538 grams of white powder solid, which is type I clopidogrel bisulfate, with a yield of 77% and a crystal form purity of 100% ( Figure 1~2 It shows that the obtained clopidogrel bisulfate is pure type I).
Embodiment 2
[0027] Put 0.551 g of clopidogrel free base and 5 ml of tetrahydrofuran into a 100 ml flask, stir at -12°C until the solution is clear, slowly add 0.175 g of concentrated sulfuric acid with a mass fraction of 98%, and react at -12°C for 1-2 hours , slowly raised the temperature to 20°C, and continued to stir and crystallize at this temperature for 10-12 hours. After the crystallization was completed, filter, wash with tetrahydrofuran, and drain as much as possible. The filter cake was vacuum-dried for 3 to 4 hours to obtain 0.521 g of white powder solid, which was type I clopidogrel bisulfate, with a yield of 73% and a crystal form purity of 100%.
Embodiment 3
[0029] Put 0.502 g of clopidogrel free base and 5 ml of tetrahydrofuran into a 100 ml flask, stir at 0°C until the solution is clear, slowly add 0.166 g of concentrated sulfuric acid with a mass fraction of 98%, react at 0°C for 1-2 hours, slowly Raise the temperature to 20°C, and continue to stir and crystallize at this temperature for 10-12 hours. After the crystallization is completed, filter, wash with tetrahydrofuran, and drain as much as possible. The filter cake was vacuum-dried for 3-4 hours to obtain 0.514 g of a white powder solid, namely type I clopidogrel bisulfate, with a yield of 79% and a crystal form purity of 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com