New method for preparing citric acid tofacitinib medicinal crystal form
A technology of tofacitinib and citric acid, applied in the field of medicine, can solve the problems of unreproducible operation, unsatisfactory purification effect, undisclosed and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
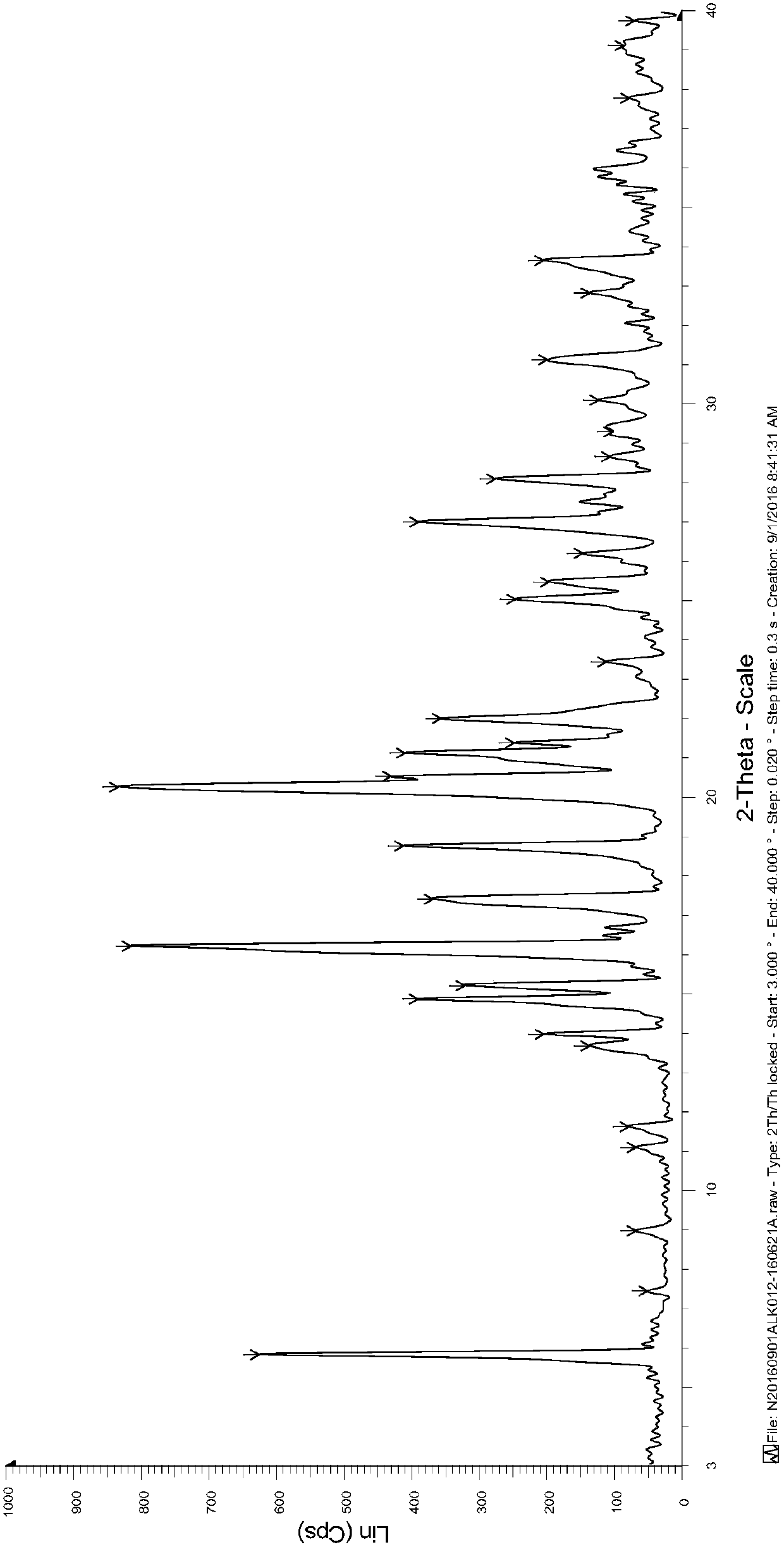
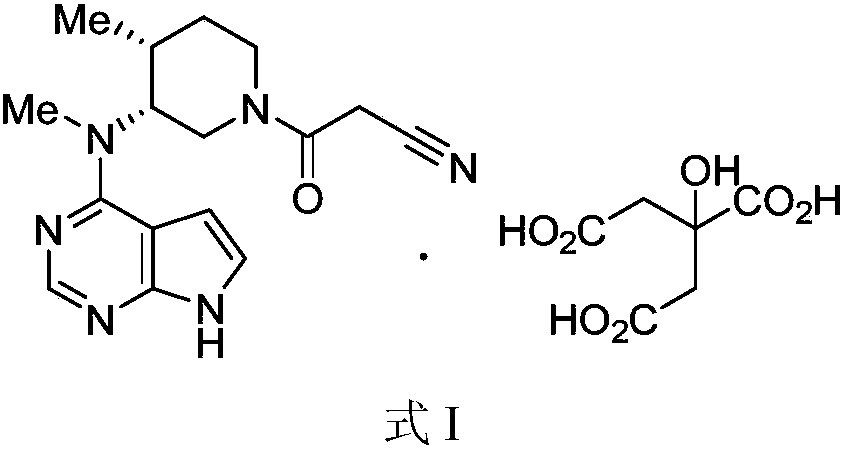
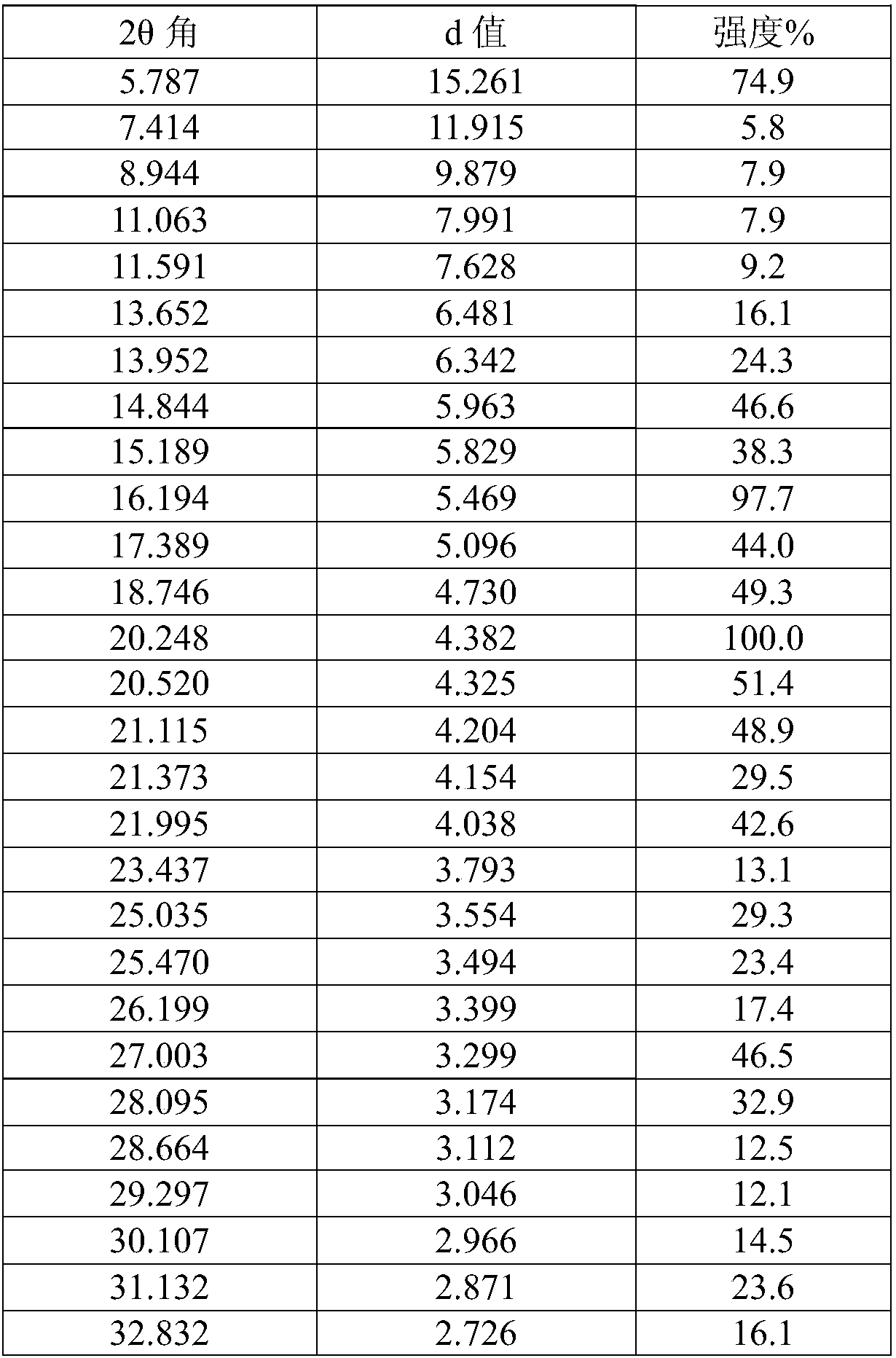
[0027] (3R,4R)-4-methyl-3-(methyl-7H-pyrrole[2,3-d]pyrimidin-4-ylamino) with a purity of 94% (normalized method) detected by HPLC -β-carbonyl-1-piperidinepropionitrile (10.00g, 32.01mmol) was dissolved in 100ml of acetone / water mixed solvent (acetone / water=9 / 1, v / v), heated to 50-60°C, and stirred until Dissolve completely. To the clear liquid was added dropwise a solution of citric acid monohydrate (7.40 g, 35.21 mmol) in water (20 ml). The resulting mixture was kept at 50-60° C. and stirred for 30 minutes, then cooled to room temperature and stirred for another 2 hours. After filtering, the filter cake was washed with acetone and dried under vacuum at 40° C. to obtain 15.00 g of white crystalline powder with a yield of 93% and a purity of 99% by HPLC (normalization method).
Embodiment 2
[0029] (3R,4R)-4-methyl-3-(methyl-7H-pyrrole[2,3-d]pyrimidin-4-ylamino) with a purity of 96% (normalized method) detected by HPLC -β-carbonyl-1-piperidinepropionitrile (5.00g, 16.01mmol) was dissolved in 30ml of ethanol / water mixed solvent (ethanol / water=9 / 1, v / v), heated to 50-60°C, stirred until Dissolve completely. To the clear solution was added dropwise a solution of citric acid monohydrate (3.70 g, 17.61 mmol) in water (10 ml). The resulting mixture was kept at 50-60° C. and stirred for 30 minutes, then cooled to room temperature and stirred for another 2 hours. After filtering, the filter cake was washed with ethanol and dried under vacuum at 40° C. to obtain 7.60 g of white crystalline powder with a yield of 94% and a purity of 99% by HPLC (normalization method).
Embodiment 3
[0031] (3R,4R)-4-methyl-3-(methyl-7H-pyrrole[2,3-d]pyrimidin-4-ylamino) with a purity of 95% (normalized method) detected by HPLC -β-carbonyl-1-piperidinepropionitrile (5.00g, 16.01mmol) was dissolved in 30ml of methanol / water mixed solvent (methanol / water=9 / 1, v / v), heated to 50-60°C, stirred until Dissolve completely. To the clear solution was added dropwise a solution of citric acid monohydrate (3.70 g, 17.61 mmol) in water (10 ml). The resulting mixture was kept at 50-60° C. and stirred for 30 minutes, then cooled to room temperature and stirred for another 2 hours. After filtering, the filter cake was washed with methanol and dried under vacuum at 40° C. to obtain 7.09 g of white crystalline powder with a yield of 88%, and the purity detected by HPLC was 99% (normalized method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com