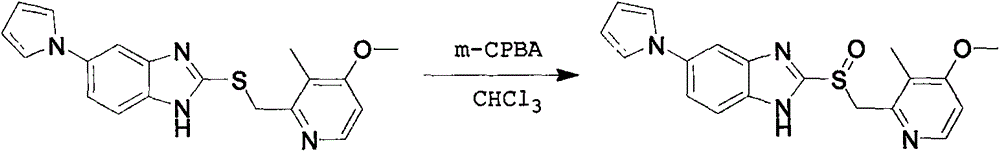
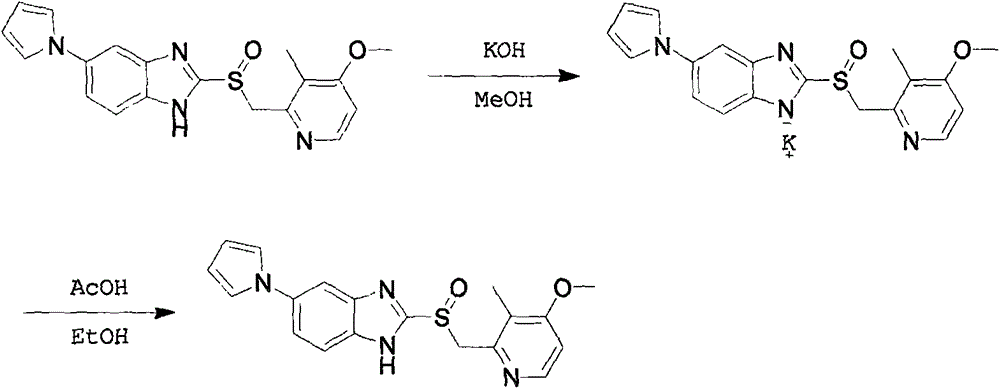
A preparing method for high-purity ilaprazole sodium
A high-purity technology of ilaprazole sodium, applied in the field of pharmaceutical preparation, can solve the problems of side reactions, difficult removal of sulfonyl peroxide, and inability to effectively remove impurities such as ilaprazole sodium salt, and achieves easy handling, The effect of simple and easy refining process and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: 5-(1H-pyrrol-1-yl)-2-[[(4-methoxy-3-methyl)-2-pyridyl]-methyl]-sulfinyl-1H-benzene Preparation of imidazole potassium salt
[0025] Add methanol (110mL) and potassium hydroxide (3.1g) to a 250mL three-necked flask, stir to dissolve and clarify, then add 5-(1H-pyrrol-1-yl)-2-[[(4-methoxy-3- Methyl)-2-pyridyl]-methyl]-sulfinyl-1H-benzimidazole (20 g), and the mixture was stirred at 25° C. for 18 hours. The mixture was filtered, the filter cake was washed with methanol (20mL*2), and the collected solid was dried at 40°C for 4 hours to obtain 5-(1H-pyrrol-1-yl)-2-[[(4-methoxy-3 -Methyl)-2-pyridyl]-methyl]-sulfinyl-1H-benzimidazole potassium salt (20.0 g), yield: 90%.
Embodiment 2
[0026] Example 2: 5-(1H-pyrrol-1-yl)-2-[[(4-methoxy-3-methyl)-2-pyridyl]-methyl]-sulfinyl-1H-benzene Preparation of imidazole sodium salt
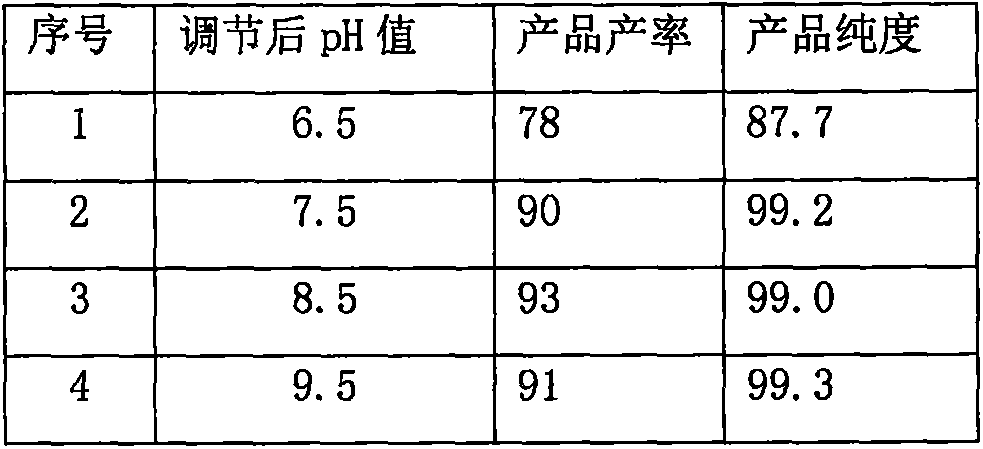
[0027] Add organic solvent A (110mL) and sodium hydroxide (2.2g) to a 250mL three-necked flask, stir to dissolve and clarify, then add 5-(1H-pyrrol-1-yl)-2-[[(4-methoxy- 3-methyl)-2-pyridyl]-methyl]-sulfinyl-1H-benzimidazole (20 g), and the mixture was stirred at a specific temperature for 18 hours. The mixture was filtered, the filter cake was washed with methanol (20mL*2), and the collected solid was dried at 40°C for 4 hours to obtain 5-(1H-pyrrol-1-yl)-2-[[(4-methoxy-3 -methyl)-2-pyridyl]-methyl]-sulfinyl-1H-benzimidazole sodium salt, specifically as shown in Table 1:
[0028] Table 1: Comparison of experimental results of organic solvent A and reaction temperature
[0029] serial number
[0030] As shown in the table above, the organic solvent A is preferably methanol or ethanol, and the reaction temperature is preferably...
Embodiment 3
[0031] Example 3: 5-(1H-pyrrol-1-yl)-2-[[(4-methoxy-3-methyl)-2-pyridyl]-methyl]-sulfinyl-1H-benzene Preparation of imidazole
[0032] Add ethanol (200mL) to a 500mL three-necked flask, 5-(1H-pyrrol-1-yl)-2-[[(4-methoxy-3-methyl)-2-pyridyl]-methyl] -Sulfinyl-1H-benzimidazole potassium salt (10g), add 10% acetic acid ethanol solution dropwise at 25°C to adjust the pH value to 9.0, let stand for 30 minutes and filter, filter cake with ethanol aqueous solution (50%, 30mL* 2) 5-(1H-pyrrol-1-yl)-2-[[(4-methoxy-3-methyl)-2-pyridyl]-methyl] after washing and drying at 30°C -Sulfinyl-1H-benzimidazole (7.4 g, white solid), yield: 82%, purity: 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com