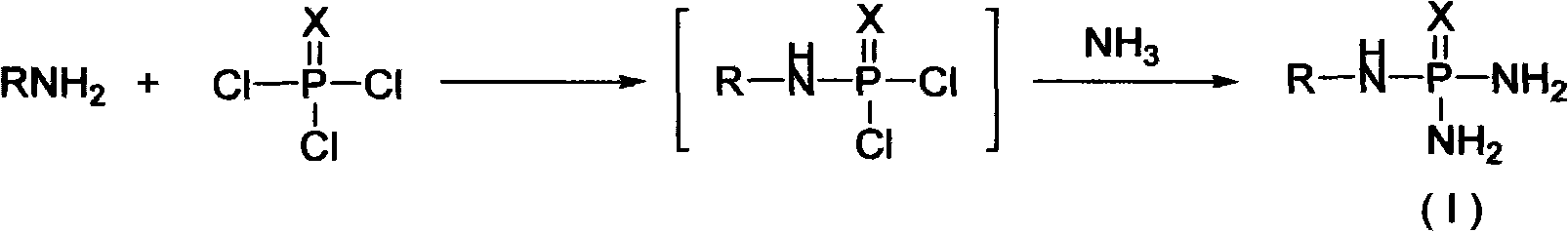Patents
Literature
109results about How to "Simple refining process" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
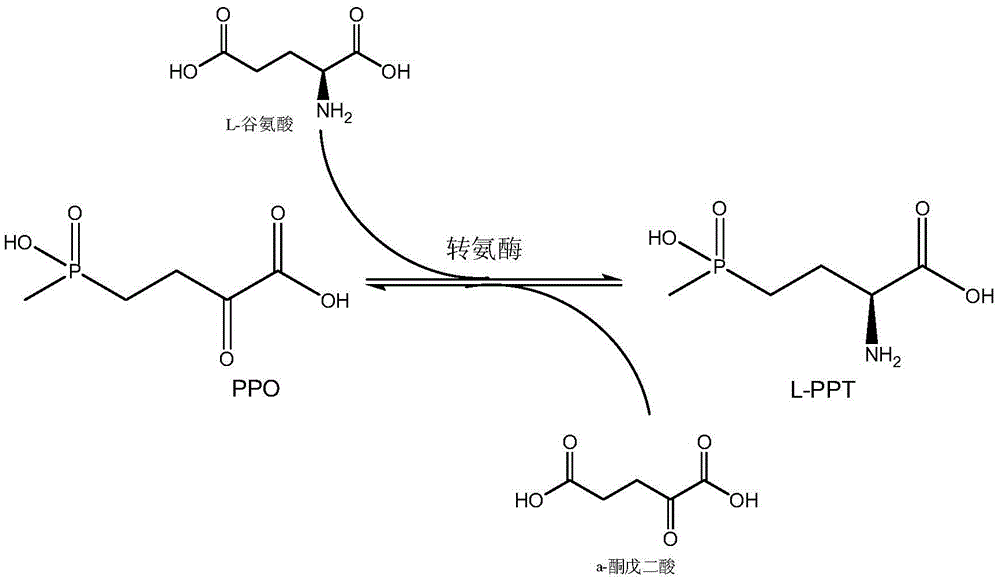
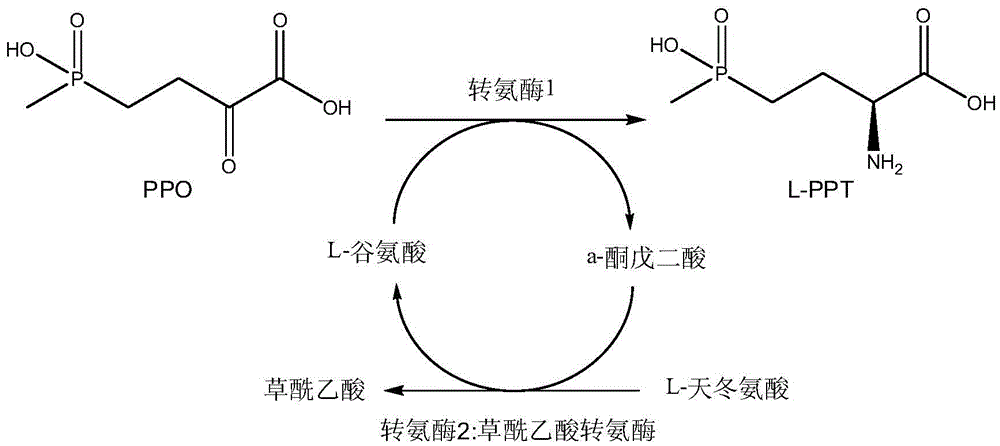
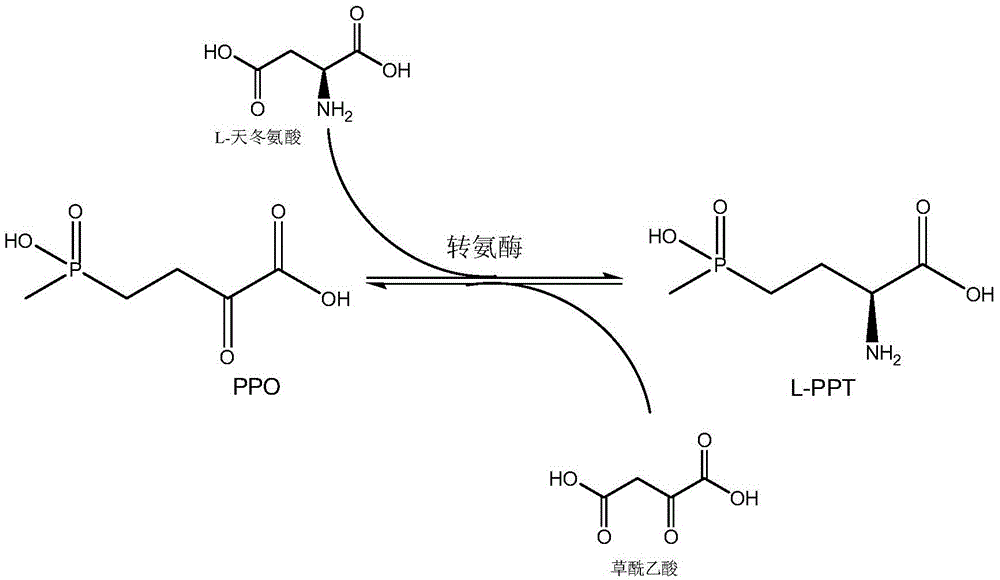
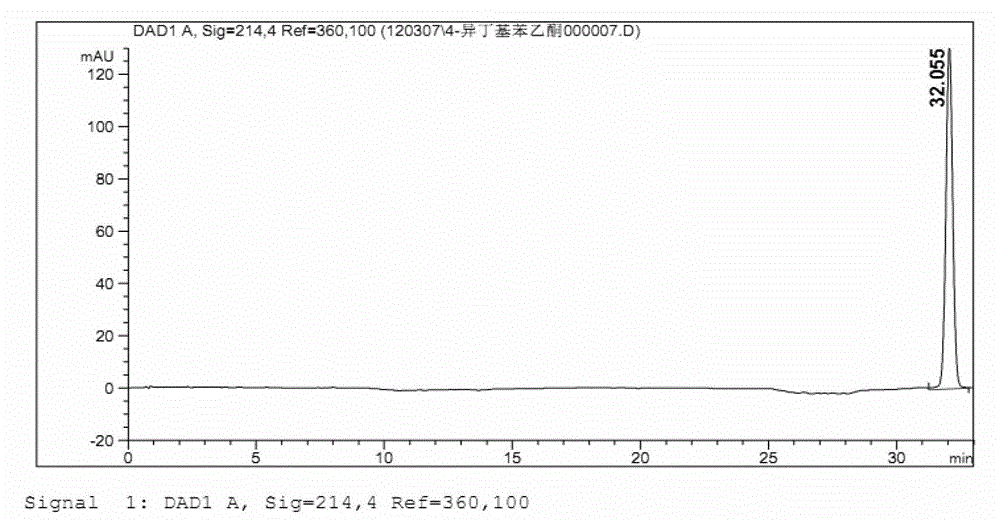
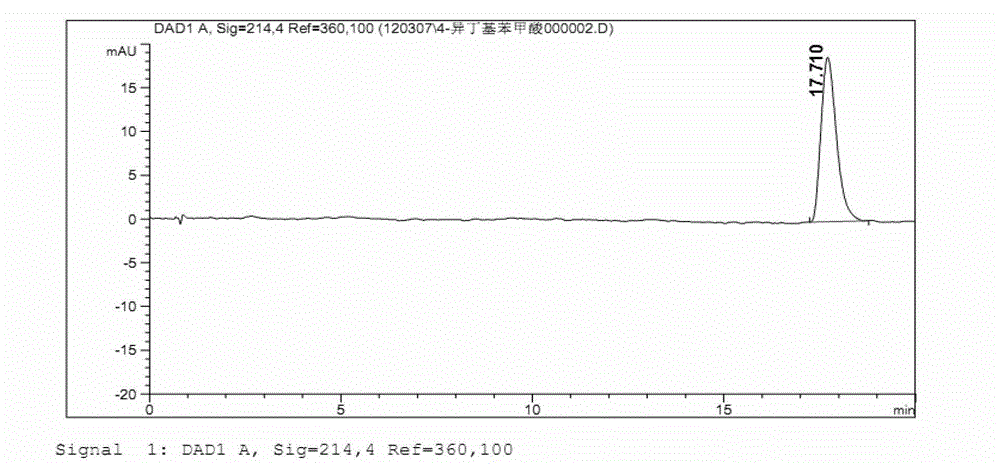
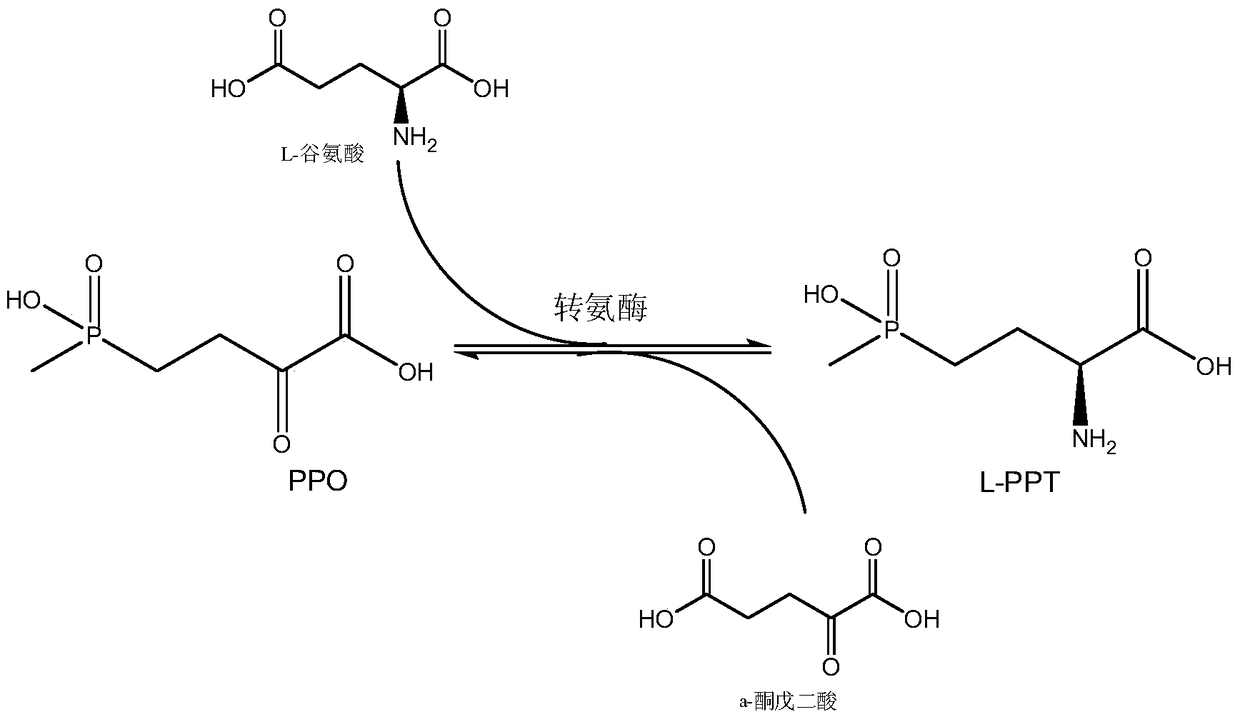
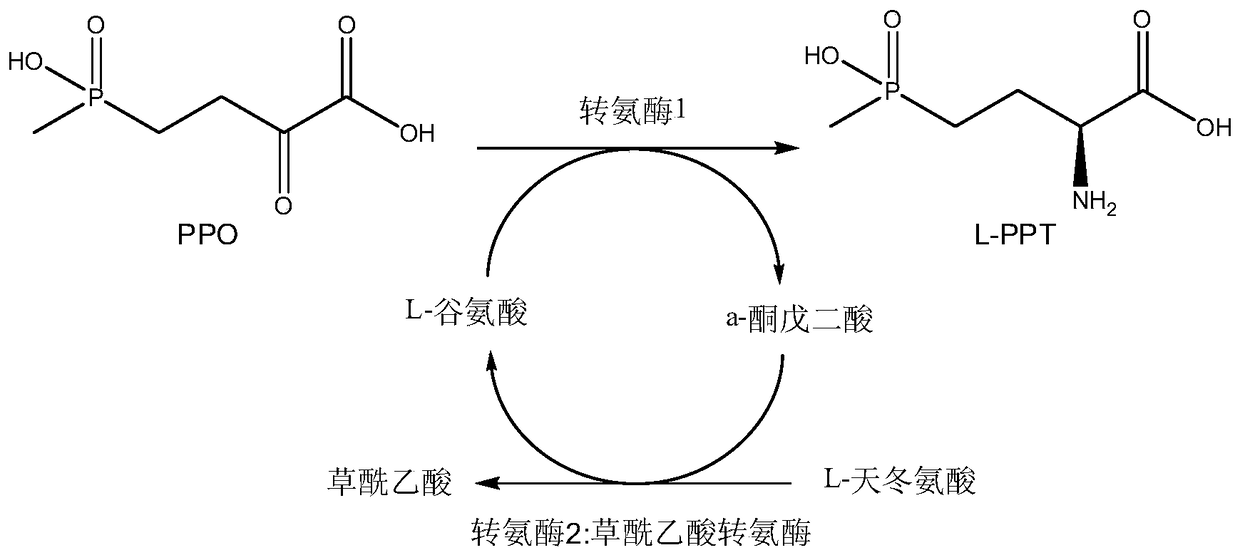
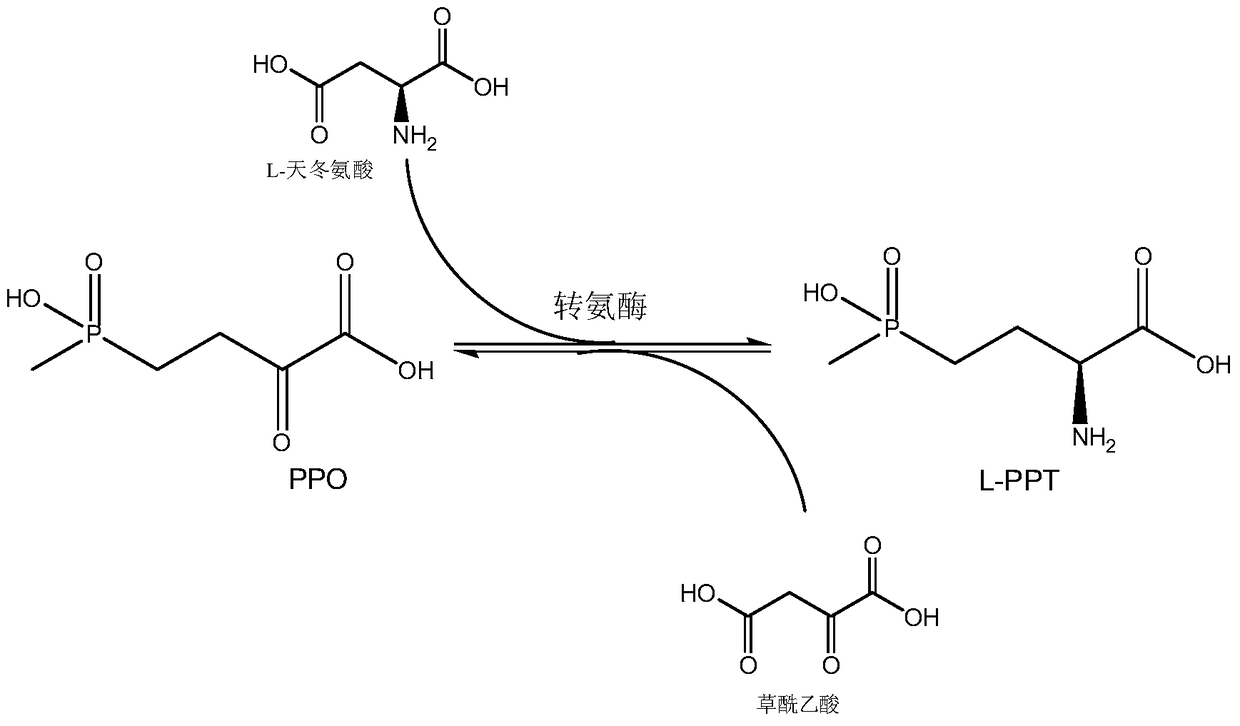
Production method of L-glufosinate
ActiveCN105603015AImprove conversion rateSimple separation processTransferasesMicroorganism based processesButyric acidHydroxy compound
The invention discloses a production method of L-glufosinate. The method includes the steps that with 2-carbonyl-4-(hydroxyl methyl phosphoryl) butyric acid and salt thereof being a substrate, isolated transaminase or a cell catalysis substrate of in-vitro expression transaminase reacts with an amino donor under the condition that the amino donor exists, so that L-glufosinate is obtained, wherein the amino donor is alanine, and the amino acid sequence of transaminase is shown in SEQ ID NO.1-3. With 2-carbonyl-4-(hydroxyl methyl phosphoryl) butyric acid and salt thereof being the substrate and alanine being the amino donor, the transamination reaction occurs through the specific transaminase catalysis substrate, the substrate can be completely converted into L-glufosinate, and the conversion rate of raw materials is high and can reach 100%.
Owner:ZHEJIANG UNIV
Method for separating ternary alloy of lead, tin and stibium
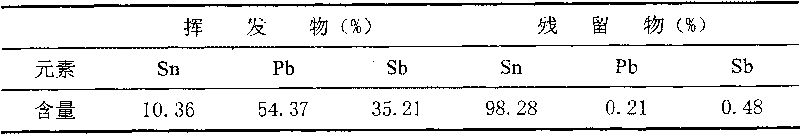
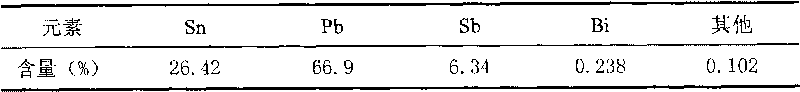
InactiveCN101696475AEasy to separateSimplify the refining processProcess efficiency improvementBoiling pointLiquid state
The invention relates to a method for separating a ternary alloy of lead, tin and stibium, which adopts a vacuum distillation method to treat the ternary alloy of lead, tin and stibium, wherein the distillation temperature is controlled at 900 to 1,200 DEG C, the distillation time is 40 to 60min and the vacuum degree is 5 to 15 Pa. The three components in the alloy are distilled in one step, then the tin of a high boiling point is kept in a liquid state, and the lead and the stibium of a low boiling point are volatilized from the alloy in a gas state so as to be separated from the liquid tin. The method can reduce the content of the lead and the stibium in the lead to be less than 1 percent, and the recovery rates of the lead, tin and stibium are over 98 percent.
Owner:KUNMING UNIV OF SCI & TECH +1
Method for preparing salidroside
ActiveCN101974045AImprove extraction efficiencySimple processSugar derivativesSugar derivatives preparationSalidrosideMicrowave
The invention provides a method for preparing salidroside, which comprises the following steps: 1) extraction: adding water into a Rhodiola crenulata (Hook. f. et Thoms.) H. Ohba medicinal material, performing microwave extraction, filtering, concentrating and obtaining concentrate; 2) purification: performing secondary alcohol precipitation of the concentrate obtained by the step 1) and obtaining filtrate; and 3) refining: loading the filtrate obtained by the step 2) on a macroporous resin, eluting, concentrating, drying and obtaining the salidroside. The preparation method of the invention has the advantages of high extraction efficiency, simple process, capability for industrial production and the like.
Owner:KANGMEI PHARMA
Method for preparing forsythiaside A
InactiveCN101215302ASimple refining processImprove extraction efficiencyEsterified saccharide compoundsSugar derivativesIonSolvent
A process for preparing forsythiaside A comprises using forsythia shell as raw material, using deionized water and water alcohol as extracting solution, passing through preliminary separation column and refining column, using deionized water and water alcohol as eluent, and then obtaining forsythiaside A after being frozen and dried, the content can reaches 85%-99%. The invention uses cheap material, no toxic and safe solution, and has simple technology, high forsythia usage and low cost, which is suitable to the industrialized production, and the extracting rate is 0.5%-2%.
Owner:SHANXI UNIV
Method for preparing hydroxyl carthamus tinctorius yellow color A
ActiveCN101215307ASimple refining processImprove extraction efficiencySugar derivativesSugar derivatives preparationAlcoholSolvent
A process for preparing hydroxyl carthamus tinctorius yellow color A comprises using dry carthanus tinctorius as raw material, using deionized water and water alcohol as extracting solution, passing through preliminary separating column and refining column, using deionized water and water alcohol as eluent, and then obtaining hydroxyl carthamus tinctorius yellow color A after frozen and dried, and the content can reach 85%-99%. The invention uses cheap material, no toxic and safe solution, and has simple technology, high carthamus tinctorius usage and low cost, which is suitable to the industrialized industry, and the extracting rate is 0.8%-1.5% according to different purify requests of hydroxyl carthamus tinctorius yellow color A for various applications.
Owner:山西华卫药业有限公司
Method for preparing m-phthaloyl chloride in high purity
ActiveCN1562946ASimple production processSimple refining processOrganic compound preparationCarboxylic compound preparationChemical reactionChloride
This invention relates to prepn. of high pureness m-benzene diformyl chloride, by using m-benzene dicarbonic acid as raw material, reacting with chlorination agent under function of catalyst, then proceeding chemical reaction, separation, refined to produce high pureness m-benzene diformyl chloride with pureness up to 99.8%.
Owner:WANHUA CHEM GRP CO LTD
Method for producing N-alkyl substituted phosphoric triamide
InactiveCN101337976AReduce energy consumptionEasy to operateGroup 5/15 element organic compoundsOrganic phosphatic fertilisersOrganic solventDesiccant
The invention provides a method for producing N-alkyl substitution phosphoryl triamide, which comprises the following steps: phosphorus chloride liquid is mixed with at least one organic solvent for precooling, then alkylamine solution is added drop by drop, and the reaction temperature ranging from 10 to 12 DEG C is kept; refluxing through heating is performed after adding drop by drop until the reaction is completed; after the reaction feed solution is washed and an organic layer is dried through dryer, ammonia gas is pumped in the next reaction; the pumping speed of ammonia gas is controlled, and the temperature of a reaction system is kept within 10 to 30 DEG C until the reaction is completed; generated solid in the reaction system is separated; the obtained organic layer is distilled to remove most solvent after being washed and separated; residue is a crude product that is N-alkyl substitution phosphoryl triamide containing a formula (1), and the crude product is recrystallized so as to obtain a fine product, the purity quotient of which is more than 98 percent. Compared with other prior arts, the method has the characteristics that the reaction condition is mild, the technology is simple, the cost is low, the purity quotient of the product is high, etc., and the method is particularly suitable for industrialized production.
Owner:张九治
Scouring method of real silk fabric
ActiveCN103469563ASpeed up refining timeLower refining temperatureBleaching apparatusAnimal fibresPre treatmentChemistry
The invention discloses a scouring method of a real silk fabric. The scouring method comprises the following steps: (1) pre-treating raw silks in an acid liquid at the temperature of 50-60 DEG C to swell the silks; (2) performing primary scouring with a scouring agent, namely a composite of an alkali scouring agent and a surfactant scouring agent; (3) performing secondary scouring, wherein a bleaching agent is added into a secondary scouring agent; and (4) after-treating, namely treating the scoured real silks from step (3) in water at 45-55 DEG C after swelling. The scouring method is simple, enhances the scouring effect of the scouring agents, and effectively solves the problem of unclean effluent of the alkali scouring agent; meanwhile, the scoured silks have the characteristics of high strength, good capillary effect, soft handfeel, excellent whiteness and low cost, and the product quality is ensured.
Owner:山东信开源科技创新发展有限责任公司
Method for removing free fatty acid in tea oil by molecular distillation
InactiveCN103113987ASimple refining processQuality improvementFatty-oils/fats refiningDistillationFatty acid
The invention provides a method for removing free fatty acid in tea oil by molecular distillation. The method comprises the steps: directly adding a tea oil sample to a sample injector of a molecular still under the conditions that molecular distillation vacuum is 0.01 to 0.05 MPa, the temperature of an evaporator is 140 to 250 DEG C, the rotating speed of a scraper blade is 100 to 200rad / min, and the sample feeding speed is 3 to 12mL / min, and the distribution thickness of the oil sample under the effect of a wiping film is 0.01-0.2mm; and under the operation state, carrying out molecular distillation so as to separate light components from heavy components according to different light and heavy molecules escaping freedom degrees, so that most of the free fatty acids and some micromolecular heavy components can be removed. By adopting the method, the shortcomings that the conventional alkali refining and deacidifying technology difficultly performs separation of the salt and the oil, and the oil consumption is high during washing can be avoided, the 'alkaline flavour' is prevented from generating, the tea oil refining process is simplified, the method is convenient and fast, and the quality of the tea oil is improved.
Owner:曹庸
Preparation method of low-VOC high-activity high-molecular-weight polyether polyol
The invention provides a preparation method of low-VOC high-activity high-molecular-weight polyether polyol. According to the method, glycerol is used as an initiator to perform polymerization reaction with propylene epoxide under the effect of a low-alkali-content catalyst; then, ethylene oxide is used for end capping; the low-alkali-content catalyzed high-activity high-molecular-weight polyetherpolyol is added into a sealed reaction kettle; deionized water and an efficient refining adsorbent are added; nitrogen replacement is performed; mixing refining and adsorption are performed at a certain temperature; next, after dewatering and filtering, a low-VOC high-activity high-molecular-weight polyether polyol product is obtained. The method provided by the invention has the advantages thatthe technical flow process is simplified; an acid neutralization process is not used; the generation of aldehydes substances in the neutralization process is avoided; by using the efficient refining adsorbent, aldehydes and other impurities generated in the production process of polyether polyol can be effectively adsorbed; finally, the goal of reducing the VOC content in the polyether polyol is achieved. The method is suitable for being applied as a preparation method of the low-VOC high-activity high-molecular-weight polyether polyol.
Owner:HANGJIN TECHNOLOGY CO LTD
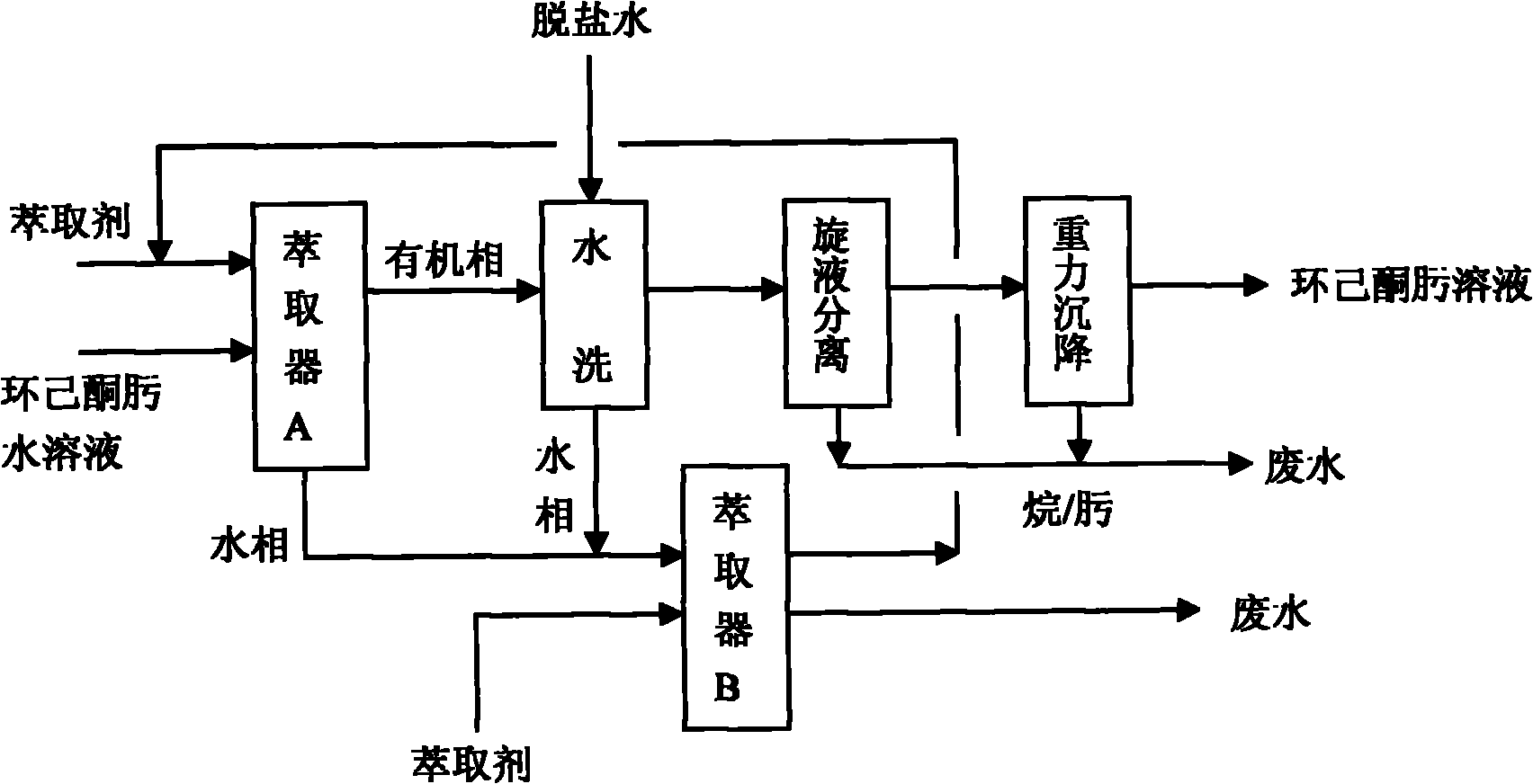
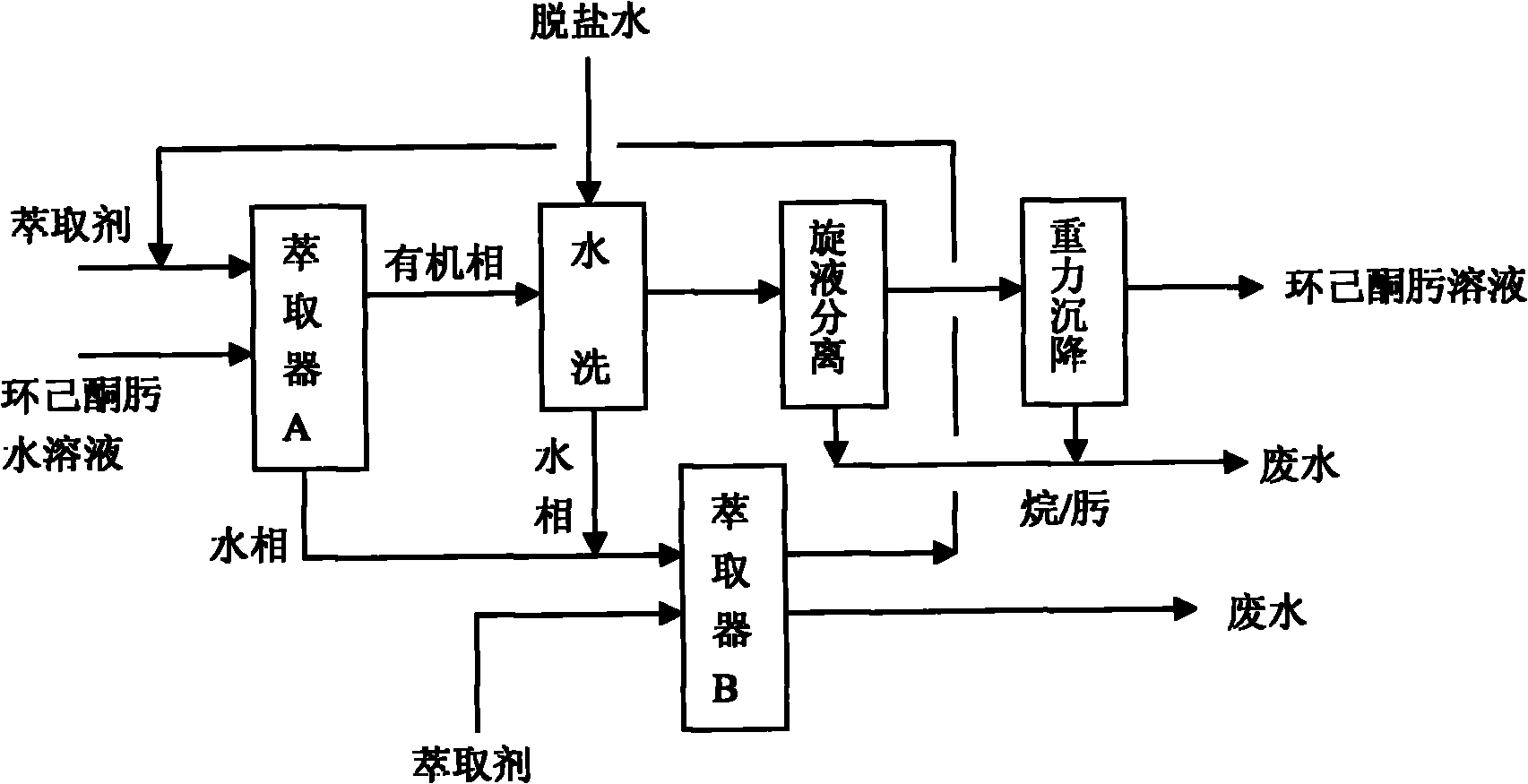
Process for extracting and washing cyclohexane oxime
ActiveCN101955445ASimple refining processFlexible refining processOximes preparationCycloneBeckmann rearrangement
The invention discloses a process for extracting and washing cyclohexane oxime. The process comprises the following steps of: extracting an extractant and aqueous solution of cyclohexane oxime in a weight part ratio of 1:1 to 2:1 at the temperature of between 45 and 55 DEG C; washing an organic phase obtained by the extraction with desalted water when the mass concentration of the cyclohexane oxime is less than 1 percent so as to remove salt ions in the cyclohexane oxime solution; mixing a water phase obtained after washing and a water phase obtained by extraction, and extracting the mixture by using a cyclohexane or hexane extractant in a weight part ratio of 0.5:1 at the temperature of between 45 and 55 DEG C so as to recycle the cyclohexane oxime in the mixture; and sequentially performing hydraulic cyclone separation and gravity settling separation on the washed organic phase when the mass concentration of the salt ions in the organic phase is less than 0.0001 percent so as to obtain the cyclohexane oxime. The process does not need a rectification process; the purity of the obtained cyclohexane oxime is high; beckmann rearrangement can be directly performed in the next process so as to prepare caprolactam; and for 100,000 t / a of cyclohexanone oxime devices, the process can save one-time equipment investment by about 20 to 30 million and can reduce energy consumption such as water, electricity, steam and the like by about 30 to 40 million.
Owner:河北美邦工程科技股份有限公司
Method for extracting and purifying artemisinin
InactiveCN103242335AHigh degree of enrichmentLow content of related substancesOrganic chemistrySilica gelSolvent
The invention discloses a method for extracting and purifying artemisinin. The method comprises the following steps of: (1) crushing artemisia apiacea and preparing artemisinin extractum; (2) melting the extractum: mixing the extractum and silica gel and drying to obtain material mixed silica gel; (3) performing wet column packing to obtain a silica gel column; (4) flatly paving the material mixed silica gel obtained in the step (2) on the silica gel column obtained in the step (3), standing, flushing the column and eluting; (5) collecting the eluent, concentrating, placing for 24 hours, crystallizing and centrifuging to obtain an artemisinin coarse product; and (6) refining the artemisinin coarse product obtained in the step (5). By adopting the method, the extraction efficiency is high, the artemisinin content is high, the using amount of solvents is small, and the method is favorable for industrial application.
Owner:SICHUAN YUTONG BIOTECH
Co-production method for extracting idesia polycarpa oil and idesia polycarpa whitening essence with multi-enzymatic and wet processes
InactiveCN105779099AIncrease production capacityImprove efficiencyFatty-oils/fats refiningFermentationCentrifugationIdesia
The invention discloses a co-production method for extracting idesia polycarpa oil and idesia polycarpa whitening essence with multi-enzymatic and wet processes. The co-production method includes pretreatment, boiling, enzymolysis, centrifugation, oil extraction and idesia polycarpa whitening essence extraction. The co-production method is a wet-process oil extraction technique, idesia polycarpa fruit are taken as raw materials, the idesia polycarpa whitening essence is extracted from pomace while the idesia polycarpa oil is extracted to turn waste into treasure, high added value of production and economic benefit are brought to planting farmers and enterprises, and the method has great practical significance and strategic significance; with the method, co-production and comprehensive utilization of oil and active ingredients are realized, the traditional oil refining process is simplified, and energy consumption is lowered; the idesia polycarpa oil prepared with the method is free of bitterness, and the problem that bitterness of the idesia polycarpa oil is hard to remove is solved; the method is simple to operate, convenient for preparation, low in cost and suitable for industrial large-scale production.
Owner:SICHUAN ZHONGHAI BIOTECH DEV CO LTD
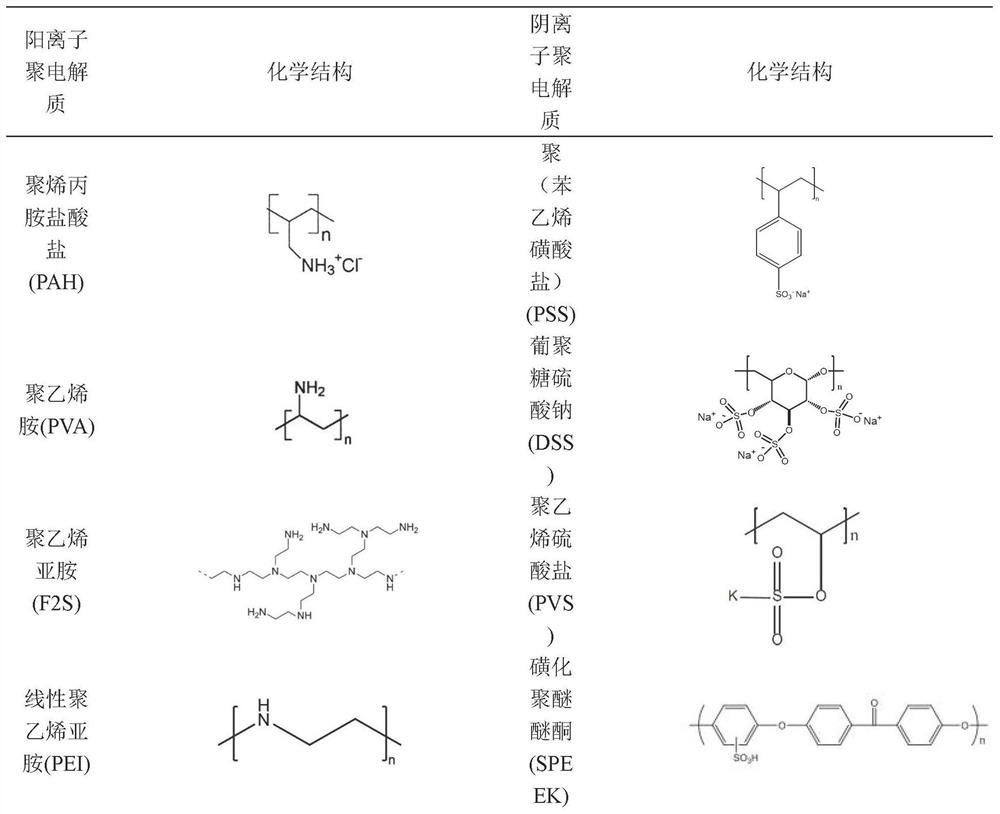
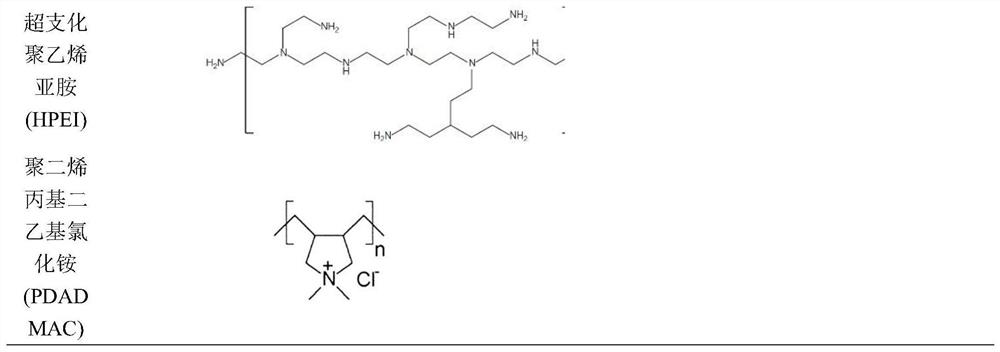
High-permeability selective acid-resistant nanofiltration membrane material as well as preparation method and application thereof
ActiveCN113332859AIncreased permeability selectivityThe film making process is simpleSemi-permeable membranesMembranesO-Phosphoric AcidUltrafiltration
The invention discloses a high-permeability selective acid-resistant nanofiltration membrane material as well as a preparation method and application thereof. The nanofiltration membrane material comprises a dense separation layer which is formed by assembling an amine and a quaternary ammonium salt polycation electrolyte and a polyanion electrolyte material with sulfonic acid groups or sulfuric acid groups layer by layer; the concentration of the polycation is 0.1-20 g / L, and the polycation is dissolved in a salt solution of 0.05-5 mol / L; the used polyanion electrolyte polymer material is a polymer material with sulfonic acid groups or sulfuric acid groups, the concentration of polyanion is 0.1-20g / L, and the polyanion is dissolved in a 0.05-5mol / L salt solution; the dense separation layer of the nanofiltration membrane is formed by layer-by-layer assembly, and has better permeation selectivity and excellent acid-resistant stability than a commercial nanofiltration membrane. The retention rate of the membrane to multivalent cations is greater than 90%, the permeability of the membrane to phosphoric acid is greater than 80%, and the permeability of phosphoric acid is one order of magnitude layer than that of that of a commercial membrane. The high-permeability selective acid-resistant nanofiltration membrane material is used in phosphoric acid refining and recycling processes, inorganic compounds in phosphoric acid-containing feed liquid are removed through pretreatment, then substances such as large particles and colloids are removed through ultrafiltration, stable operation of a nanofiltration membrane process is achieved, high-purity phosphoric acid of different grades is obtained, and the high-permeability selective acid-resistant nanofiltration membrane material has wide application in the aspects of phosphoric acid refining and phosphoric acid recycling.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Desulfurization deordorization refining method of crude sulfate turpentine
ActiveCN104449395ASimple refining processReduce energy consumptionTurpentine spiritsMolecular sieveSorbent
The present invention provides after-oxidation waster-washing separation and adsorption combined process so as to achieve efficient refining of crude sulfate turpentine. According to the present invention, the oxidant is a sodium hypochlorite solution with an effective chlorine content of 3-10%, and the adsorbent is a mixture of modified active carbon and a water absorption molecular sieve; the key of the specific refining method is that the primary after-oxidation waster-washing separation is performed, and then adsorption with the adsorbent is performed to remove irritating odor, such that the organic sulfur content in the crude sulfate turpentine can be efficiently removed, and the total sulfur content can meet the 10 ppm standard required by the industrial application requirement; and with the excessive oxidation, the application of the modified active carbon and water absorption molecular sieve mixture as the adsorbent, and other means, the refining process is simplified, the energy consumption is reduced, and the refining cost is saved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Industrialized production method of 3,4-dimethylpyrazole and phosphate thereof
ActiveCN102311388AHigh purityReduce the difficulty of operationOrganic chemistryPhosphateDistillation
The invention relates to an industrialized production method of 3,4-dimethylpyrazole and a phosphate thereof, and mainly solves problems of rigor production technology and expensive raw materials of a prior synthetic method. The technology of the invention is easily operated, at low costs and with high yield; reaction conditions are apt to realization of large-scale industrialized production. A technical scheme of the invention is as below: the industrialized production method of 3,4-dimethylpyrazole and the phosphate thereof comprises the steps that 2-butanone reacts with solid formaldehyde in an alkaline solution, and 3-hydroxymethyl-butanone is obtained after distillation and separation; the 3-hydroxymethyl-butanone reacts with hydrazonium salt under an acid condition to obtain 3,4-dimethylpyrazole, which reacts with phosphoric acid to form a salt of 3,4-dimethylpyrazole phosphate. The 3,4-dimethylpyrazole phosphate obtained by the invention is an efficient nitrifying ferment inhibitor.
Owner:林文斌
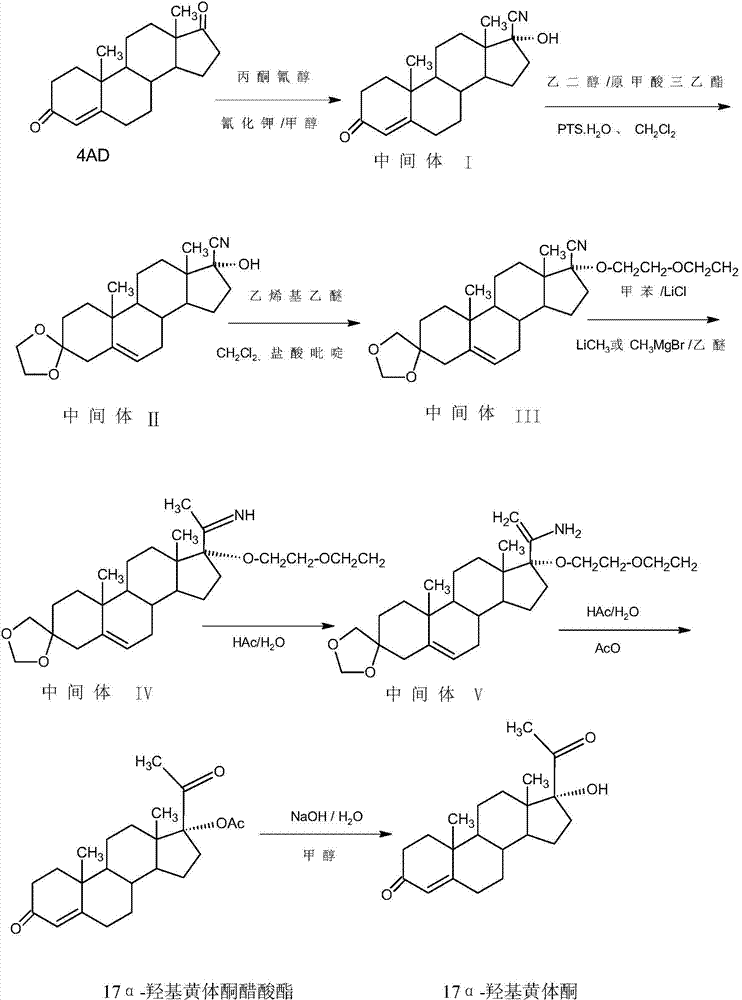
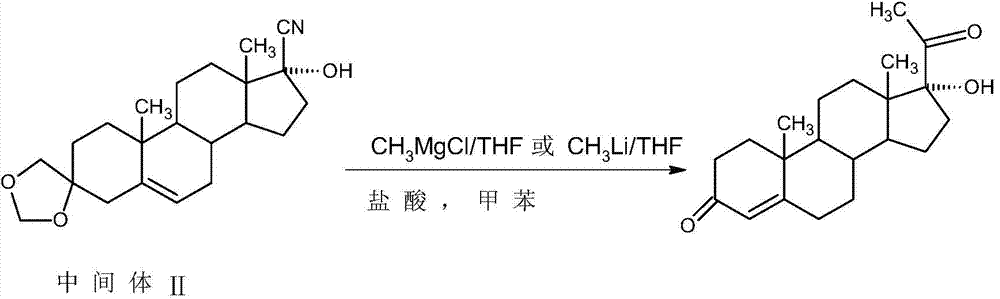
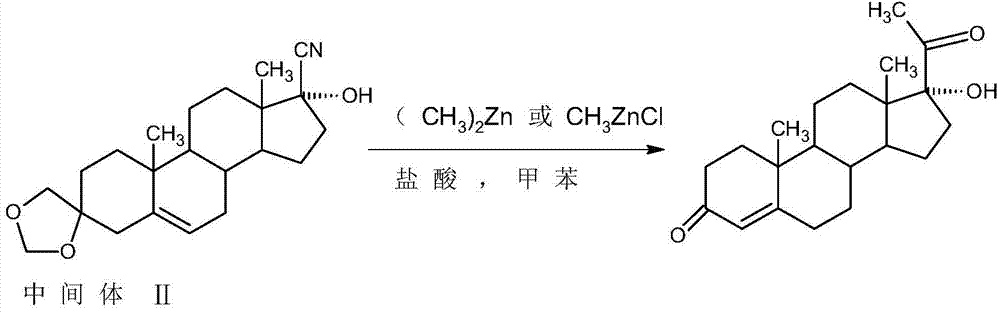
Method for preparing 17alpha-hydroxyprogesteron
The invention relates to a method for preparing 17alpha-hydroxyprogesterone. The17alpha-hydroxyprogesterone is prepared by taking 17beta- cyano-5-androstene-17-ol-3,3-diethylene ketal (referred as an intermediate II) as a raw material and dimethylzinc or methylzinc chloride as a reagent; the content of the 17alpha-hydroxyprogesterone by HPLC is above 99.5% and the weight yield is 83-87%. The method comprises the following steps of dissolving the intermediate II in an organic solvent, adding lithium chloride as a catalyst, stirring, raising the temperature to 40-80 DEG C, dropwise adding a toluene solution of dimethylzinc or methylzinc chloride of which the concentration is 2M, and continuing to complete the reaction; and then adding an ammonium chloride solution of which the concentration is 25% to destruct an organic zinc reagent, separating the aqueous layer out and extracting, merging the organic layer and the extract and concentrating the solvent to near dryness, and then adding lower alcohol, stirring, raising the temperature to 40-60 DEG C, adding the acid of which the concentration is 2M, hydrolyzing, adjusting the pH value with a weak base after the reaction is completed, evaporating 90% of the solvent out, adding tap water, cooling and crystallizing to obtain a crude 17alpha-hydroxyprogesterone product; and then carrying out reflux decolorizing on the crude product with activated carbon by virtue of alcohol, and refining to obtain the commercial grade 17alpha-hydroxyprogesterone. The 17alpha-hydroxyprogesterone produced by the method disclosed by the invention has the advantages of good purity and high yield and is economic and environment-friendly, and the solvent can be recycled.
Owner:HUNAN KEREY BIOTECH
Device for gathering liquid drops in metal slag by adding pulsed electric field
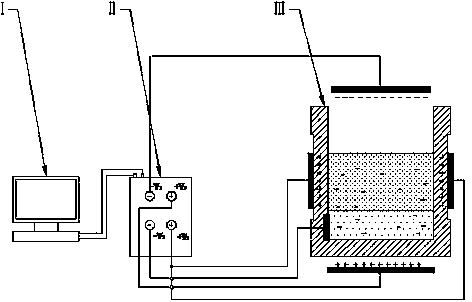
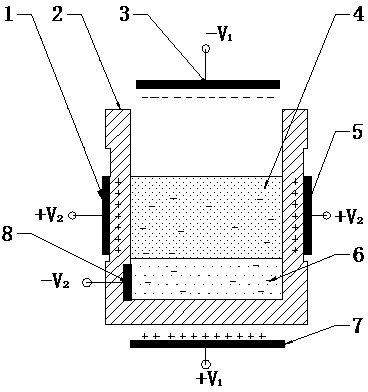
InactiveCN103966454AAchieve the purpose of separationEasy to separateRecycling and recovery technologiesProcess efficiency improvementCapillary actionMetal recycling
The invention discloses a device for gathering liquid drops in metal slag by adding a pulsed electric field. The device comprises a controller, a pulsed power supply and a reaction furnace, wherein a series of electrode plates are inlaid on a furnace body and correspondingly connected with electrode ends of the pulsed power supply, and an electric field generation system is formed; a slag-metal system in the reaction furnace is under the action of the electric field, the pulsed power supply is controlled to charge the slag-metal system, and the pulsed electric field is generated; and after charging of the slag-metal system is completed, the metal liquid drops in the slag-metal system move in the electric field action zone under the action of the electric field, and the metal liquid drops diffused in the metal slag are gathered and grown and fall into the bottom of the slag-metal system to form a metal liquid tank, so that a metal liquid is recovered. According to the device, the metal liquid drops move and are gradually gathered under the electric capillary action by adjusting electric field force of the metal liquid drops in the slag during charging, so that residual metal is separated from the slag, the metal recovery rate is increased, the process is simple, the material cost is low, and the device is suitable for industrial application.
Owner:SHANGHAI UNIV
Preparation method of non-edible animal and plant crude oil refined oil
The invention relates to a preparation method of a non-edible animal and plant crude oil refined oil. The method includes: filtering squeezed or extracted crude oil, removing solid particle impurities, conducting heating to 40-80DEG C; under stirring, adding a conversion-complexing agent accounting for 1-3% of the mass of the crude oil, conducting constant temperature stirring for 50-90min, employing centrifugal separation at the end of reaction to obtain degummed oil and oil saponins; dehydrating the degummed oil under a vacuum degree of 0.07-0.09MPa and a temperature of 95-105DEG C for 15-25min, heating the dehydrated oil to 90-110DEG C, adding 1-4% of an adsorbent, performing constant temperature stirring for 10-30min, and conducting filtering to obtain the refined oil. While removing phosphorus, metals and other impurities efficiently, the method maintains the acid value of the oil constant, thereby reducing loss and improving the refined oil yield. The method has the characteristics of simple process, low energy consumption, no wastewater discharge, and high stability, etc.
Owner:PETROCHINA CO LTD
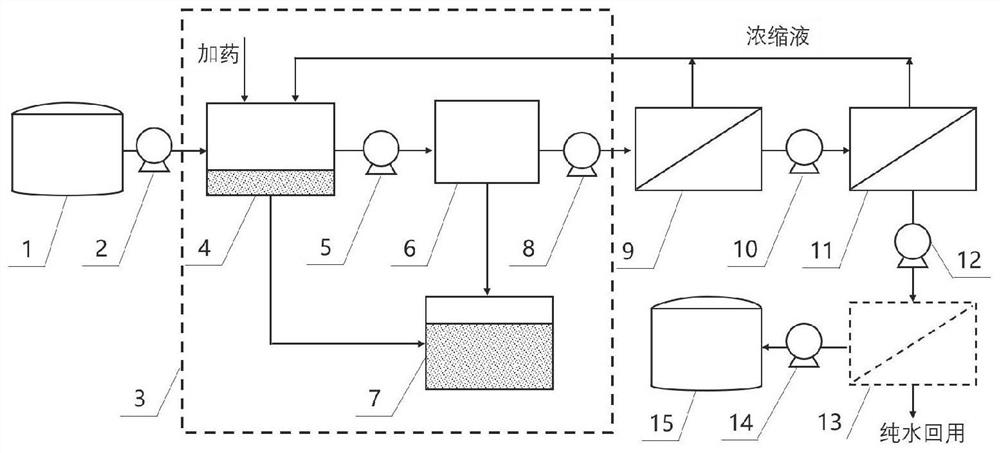
Bittern or seawater ultrafiltration pre-treatment process and system
ActiveCN104211203AReduce refining costsSimple refining processReverse osmosisWater/sewage treatment bu osmosis/dialysisUltrafiltrationSeawater
The invention discloses a bittern or seawater ultrafiltration pre-treatment process and system. The bittern or seawater ultrafiltration pre-treatment process and system can provide filtered water meeting water feeding requirements of nanofiltration. Every process cycle of the bittern or seawater ultrafiltration pre-treatment process sequentially comprises a filtering step, a water producing step, a cleaning step and a discharging step, wherein the cleaning step comprises processes including gas wiping, gas-water backwashing and gas exhausting. Bittern or seawater is filtered through a cartridge filter and precisely-filtered liquor is filtered through a ultrafilter membrane filtering device to produce water; the gas exhausting process comprises that, during a membrane module exhaust process, oilless compressed air is inlet to the water inlet side of membrane yarns, and compressed air and water are mixed and oscillated to act on the surface of the membrane yarns; during the gas exhaust process, the water level decreases continuously, the gas-water washing strengths of the cross sections at different horizontal heights change, so that the membrane yarns can be cleaned from top to bottom. The bittern or seawater ultrafiltration pre-treatment process enables the bittern and seawater to be treated in a membrane filtering mode, thereby reducing the purification cost and simplifying the purification process, and through stable water output, prolongs the service life of nanofiltration membranes and guarantees stable operation of a nanofiltration system.
Owner:TIANJIN MOTIMO MEMBRANE TECH +1
Refining method of ibuprofen
InactiveCN102875360ASimple refining processEasy to operateCarboxylic compound separation/purificationActivated carbonReflux
The invention discloses a refining method of ibuprofen, particularly relating to a refining process of the ibuprofen, impurity limit and quality comparison with the commercially available ibuprofen at present, and belonging to the technical field of medicine. The ibuprofen is mainly dissolved by using an organic solvent, active carbon is added for reflux decolorizing and then solution is crystallized, filtered, ground, dried and weighed to obtain a finished product, i.e., refined ibuprofen. According to the product prepared by the invention, the impurity content of the ibuprofen can be strictly controlled within a certain range, and requirements of all formations including injection can be met, so that adverse reaction produced to a human body can be avoided to the greatest extent and the safety is higher during intravenous drip and other administration approaches of a patient. The refining process of the ibuprofen has the advantages of simplicity, easiness in operation, high efficiency and capability of preparing a product with stable quality.
Owner:WUXI XINRENTANG PHARMA TECH
A kind of production method of L-glufosinate-ammonium
ActiveCN105603015BImprove conversion rateSimple separation processTransferasesMicroorganism based processesButyric acidHydroxy compound
Owner:ZHEJIANG UNIV
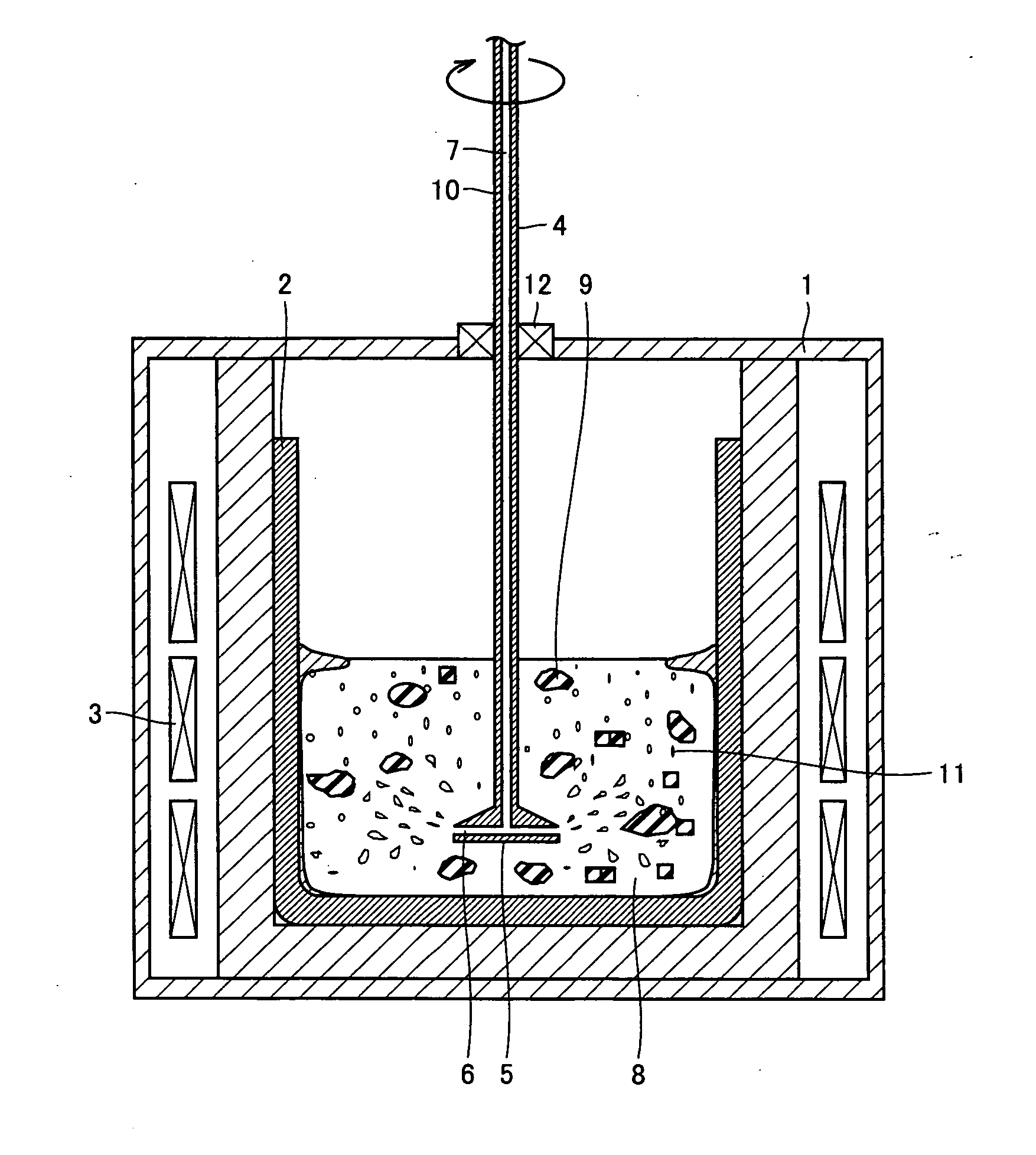
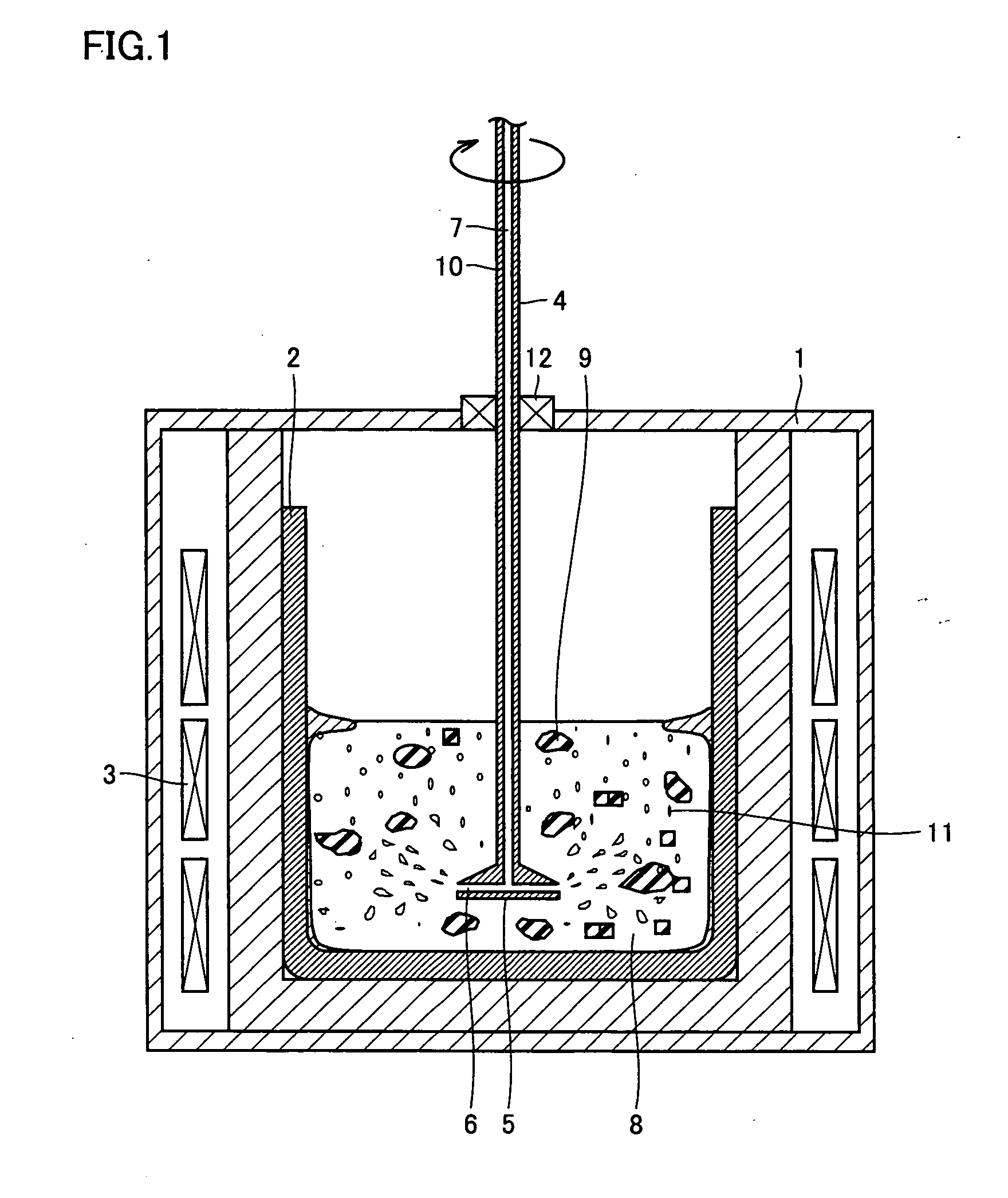
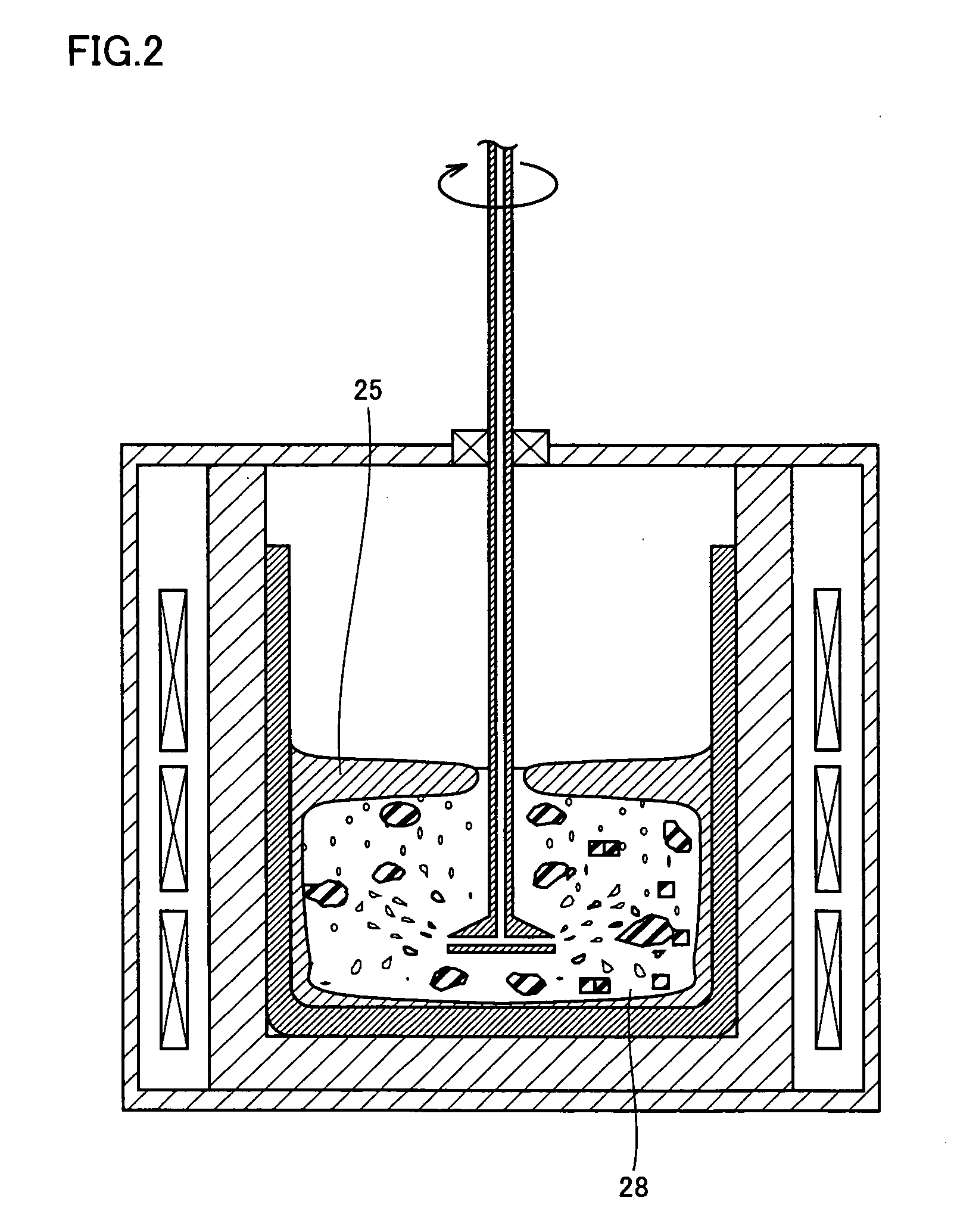
Method For Refining Silicon And Silicon Refined Thereby
In order to provide silicon for solar batteries inexpensively by efficient refining and without lowering the refining rate, the present invention is directed to a method for refining molten silicon containing an impurity element. According to one aspect, the method includes the steps of: bringing a refine gas containing a component that reacts with the impurity element into contact with the molten silicone, thereby removing a product containing the impurity element from the molten silicon; and bringing a process gas, having small reactivity with the molten silicon, with the molten silicon, thereby removing a product generated by reaction of the molten silicon and the refine gas.
Owner:SHARP KK
Control method for heavy fragrant plant oil acid value
InactiveCN1616616ALow acid valueSimple refining processFatty-oils/fats refiningFatty-oils/fats productionFlavorNatural product
The present invention is control method for acid value of strong fragrant vegetable oil. The technological process includes fully moistening vegetable oil seed with composite antioxidant aqua comprising with vitamin E, tea polyphenol, carotenoid, etc.; steaming or frying and squeezing; processing obtained vegetable oil with solid processing agent for 0.5-2 hr, and filtering to obtain strong fragrant vegetable oil. The process can lower the acid value extremely, simplify the vegetable oil refining process, maintain the original flavor and color and maintain the natural product characteristic of strong fragrant vegetable oil.
Owner:GUANGXI UNIV
Special scouring agent for silks
ActiveCN103469565ASimplify the refining processImprove refining effectAnimal fibresDefoaming AgentsChemistry
The invention discloses a special scouring agent for silks. The special scouring agent contains the following components in parts by weight: 40-60 parts of a surfactant, 30-50 parts of sodium oleate, 15-20 parts of a buffering agent, 10-20 parts of a complexing agent, 5-7 parts of a dispersing agent and 3-5 parts of an antifoaming agent. An alkali scouring agent and a surfactant scouring agent are effectively combined and simultaneously are combined with other auxiliaries such as the antifoaming agent, the complexing agent and the dispersing agent, so that the special scouring agent for silks play the roles of the alkali scouring agent and the surfactant scouring agent furthest, a scouring technology is simplified, the scouring effect of the special scouring agent is enhanced, the problem of unclean effluent of the alkali scouring agent is effectively solved, meanwhile, the scoured silks have the advantages of high strength, good capillary effect, soft handfeel, excellent whiteness and low cost, and the product quality is ensured.
Owner:山东信开源科技创新发展有限责任公司
Method for preparing vanadium-containing sponge titanium
The invention belongs to the field of titanium metallurgy, and particularly relates to a method for preparing vanadium-containing sponge titanium. The invention aims to provide a method for preparingvanadium-containing sponge titanium, the method comprises the following steps that Ticl4 with high vanadium content without vanadium removal by aluminum is distilled, refining and impurity removal, then directly introducing into a magnesium metal melting system, vanadium-containing sponge titanium is prepared through alkali metal reduction and vacuum distillation process under the protection of inert gas, and the vanadium-containing titanium sponge can be used as a raw material for preparing vanadium-containing titanium alloy. According to the method for preparing vanadium-containing sponge titanium, high-valence vanadium elements in Ticl4 can be directly recycled, the refining process flow in the Ticl4 production process is simplified, a special raw material is provided for the vanadium-titanium alloy, and the method has an important practical significance on the development of the titanium alloy.
Owner:PANZHIHUA IRON & STEEL RES INST OF PANGANG GROUP
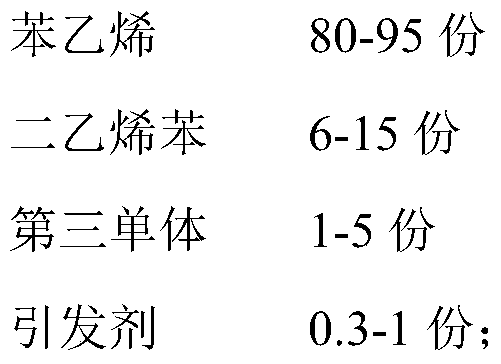
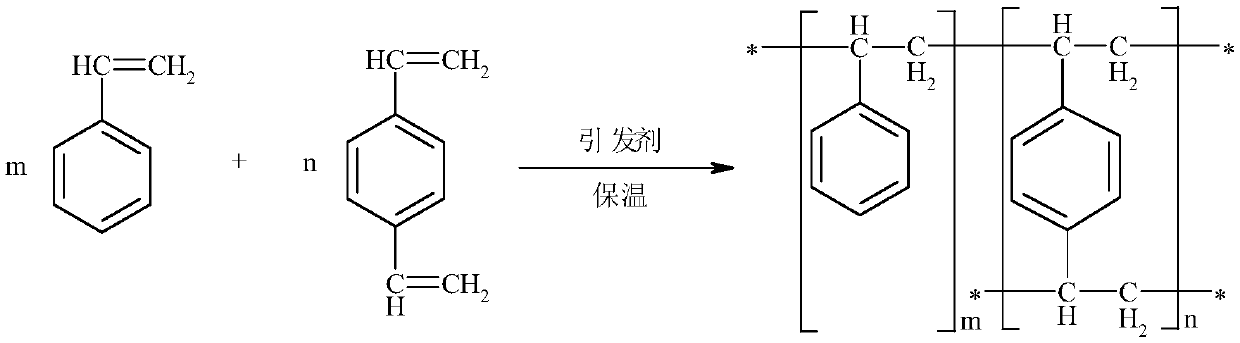
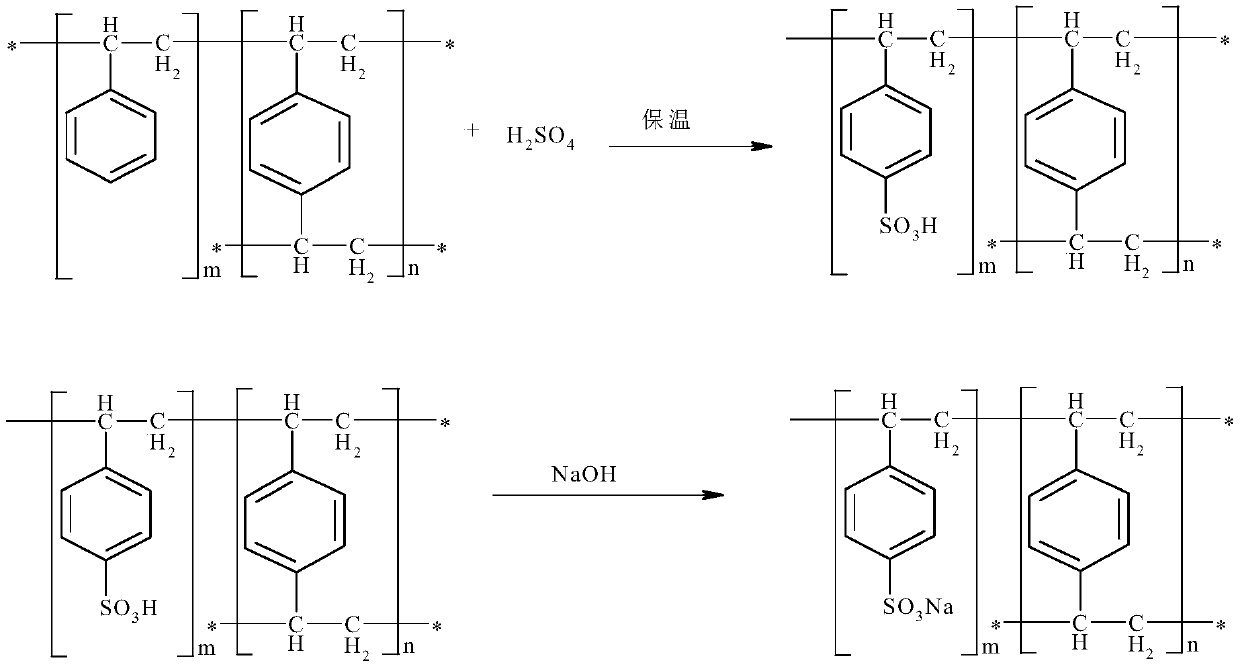
Preparation method of solvent-free gel type styrene cation exchange resin
InactiveCN111040065AImprove securityReduce refining treatment costsCation exchanger materialsPolymer scienceDivinylbenzene
The invention belongs to the technical field of cation exchange resins, and particularly relates to a preparation method of a solvent-free gel type styrene cation exchange resin. The method comprises:carrying out suspension polymerization on styrene, divinylbenzene and a third monomer under the action of an initiator to prepare white balls; and carrying out a sulfonation reaction on the white balls and sulfuric acid, and carrying out post-treatment after the reaction is finished to obtain the solvent-free gel type styrene cation exchange resin. According to the preparation method of the solvent-free gel type styrene cation exchange resin, a white ball swelling reagent 1,2-dichloroethane is not used, so that the safety of a resin product is improved, the refining treatment process is simplified, and the refining treatment cost of an enterprise is reduced.
Owner:山东德川化工科技有限责任公司
Production method for high-quality vegetable oil
ActiveCN107384579AImprove cleaning efficiencyReduce energy consumptionFatty-oils/fats refiningFatty-oils/fats productionMicrowaveVegetable oil
The invention discloses a production method for high-quality vegetable oil. The method comprises the following steps: separating non-oilseed impurities by integrating screening, winnowing, magnetic separation, color sorting and water separation devices, thereby acquiring clean fresh oilseeds; using hot air for preheating the oilseeds to 40 DEG C-55 DEG C, starting microwave processing and ending when the temperature is increased to 130 DEG C-170 DEG C; performing micro-mist jetting treatment and then performing wet film treatment, wherein the treatment time is 20min-35min and the temperature of the oilseeds after tempering is 40 DEG C-60 DEG C; adopting a screw oil press for squeezing the oilseeds under low temperature, thereby acquiring squeezed oil and squeezed cake; and heating the squeezed oil to 20 DEG C-50 DEG C; adding sodium bentonite, crystallized soda and a xanthan gum complexing agent in turn while stirring, filtering and separating. The production method has the advantages of short oil-preparing process chain, high oil-preparing efficiency, high product quality, low energy consumption and refining consumption, environmental protection, and the like.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
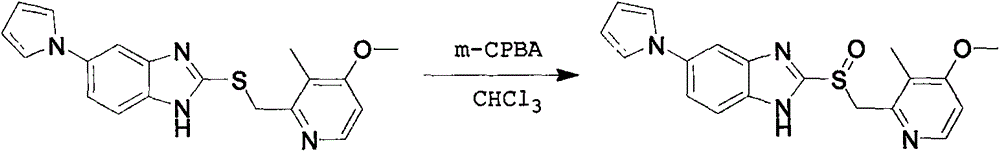
A preparing method for high-purity ilaprazole sodium
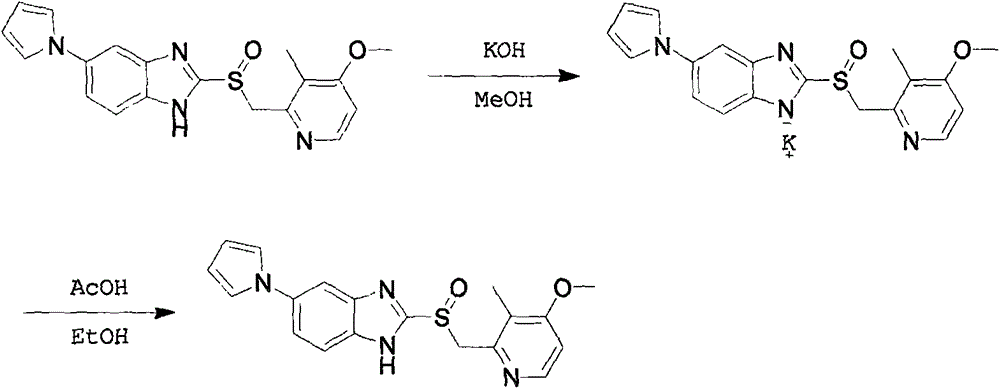
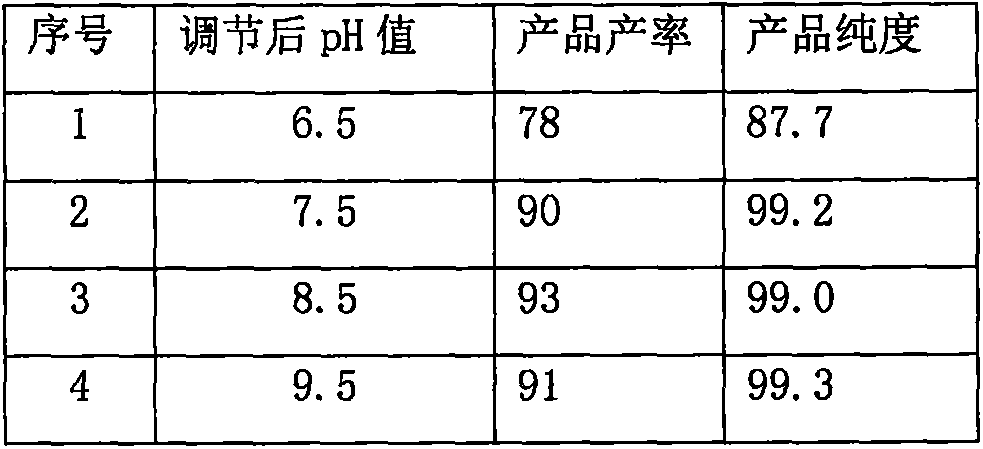
InactiveCN106045975AEfficient removalNo pollution in the processOrganic chemistryOrganic solventSolvent
A preparing method for high-purity ilaprazole sodium is provided. The method includes 1) dissolving a 2-[(4-methoxy-3-methylpyridin-2-yl)methyl]sulfinyl-5-(1H-pyrrol-1-yl)-1H-benzimidazole crude product into an organic solvent, adding an inorganic alkali to form a salt, and filtering to obtain a 2-[(4-methoxy-3-methylpyridin-2-yl)methyl]sulfinyl-5-(1H-pyrrol-1-yl)-1H-benzimidazole salt (A), 2) dissolving the A into an organic solvent, adjusting pH with an acid until reaching alkalescence to prepare high-purity 2-[(4-methoxy-3-methylpyridin-2-yl)methyl]sulfinyl-5-(1H-pyrrol-1-yl)-1H-benzimidazole (B), and 3) reacting the prepared B with a sodium-containing compound in a water-containing solvent to prepare the ilaprazole sodium.
Owner:LIVZON PHARM GRP INC +1
Squeezing preparation method of 6S1D soybean oil
PendingCN105400592AImprove oil yieldColor SuppressionFatty-oils/fats refiningFatty-oils/fats productionMetalSoybean oil
The invention discloses a squeezing preparation method of 6S1D soybean oil. The method comprises the following steps: 1, preprocessing soybeans; 2, squeezing the preprocessed soybeans; 3, degumming: adding an acid solution accounting for 1-10% of the mass of crude oil to the crude oil obtained after squeezing, and stirring for 20-40min; 4, precipitating; 5, filtering 5 times; and 6, carrying out ultraviolet irradiation. Phosphatide, tocopherol, metal ions and other components in soybeans, and extraneous factors (temperature, illumination and air) and other factors possibly causing oil color reversion, are mainly investigated in the invention to find out main reasons for causing the color reversion of the soybean oil in the purifying process. Compounded acid solution degumming treatment substantially reduces the phosphorus content of oil, so degummed oil with low phosphorus content, low acid valence and transparent and bright color is obtained, and the color reversion of the soybean oil is effectively inhibited; and the method has the advantages of avoiding of severe damages of present soybean oil pressing technologies to unsaturated fatty acids, VE and other nutritional components, high soybean oil yield and reduction of the environment pollution.
Owner:SHANGHAI TAOHONG CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com