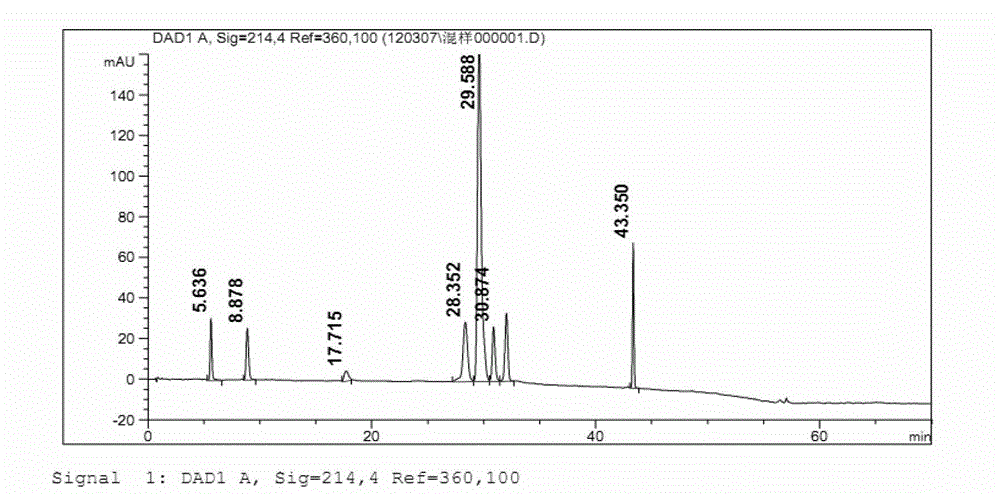
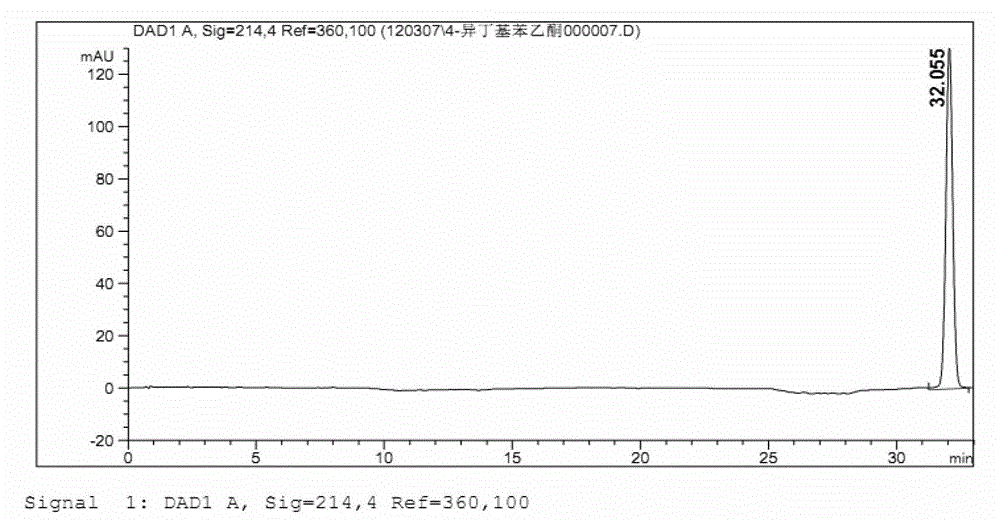
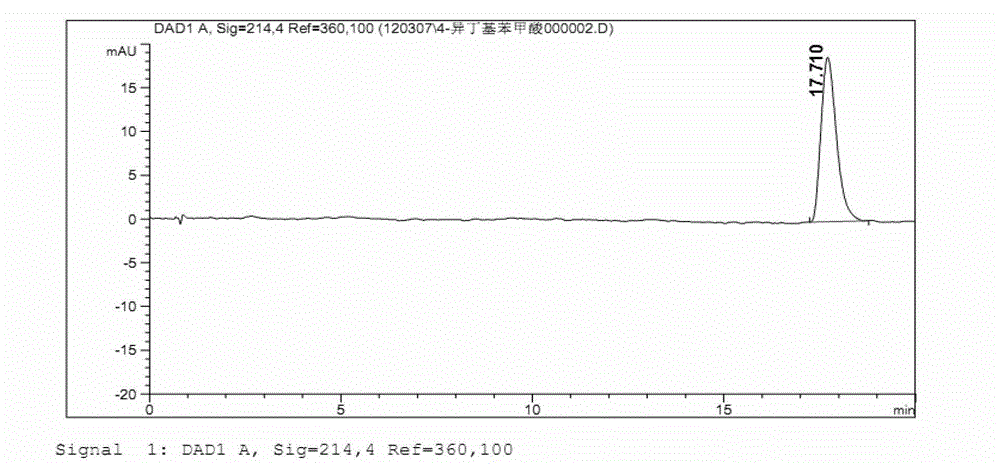
Refining method of ibuprofen
A refining method and technology of preparation steps, applied in the field of medicine, can solve the problems of not being able to reach the injection level, unknown side effects, many adverse reactions, and high impurity content, and achieve the effects of avoiding adverse reactions, easy operation, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Refining process of ibuprofen:
[0029] (1) Precisely weigh 40.00g of ibuprofen, put it in a 500mL round bottom flask, add 320mL of petroleum ether, and shake to dissolve completely;
[0030] (2) In the dissolved ibuprofen petroleum ether solution, add 0.05% activated carbon accounting for the mass volume ratio of the total solution;
[0031] (3) Heating in a water bath to 75°C for reflux reaction for 40 minutes;
[0032] (4) Filter out the activated carbon while it is hot;
[0033] (5) Allow the obtained filtrate to stand at room temperature and then crystallize overnight at -7°C;
[0034] (6) Remove petroleum ether by filtration to obtain ibuprofen filter cake;
[0035] (7) Grind the above filter cake into powder, dry in vacuum at 50°C for 3 hours and weigh to obtain the final product of ibuprofen.
Embodiment 2
[0037] Refining process of ibuprofen:
[0038] (1) Precisely weigh 80.00g of ibuprofen, place it in a 1000mL round bottom flask, add 640mL of 70% ethanol solution, and shake to dissolve completely;
[0039] (2) Add 0.2% activated carbon to the dissolved ibuprofen 70% ethanol solution;
[0040] (3) Heating in a water bath to 65°C for reflux reaction for 30 minutes;
[0041] (4) Filter out the activated carbon while it is hot;
[0042] (5) Allow the obtained filtrate to stand at room temperature and then crystallize overnight at -7°C;
[0043] (6) Remove 70% ethanol solution by filtration to obtain ibuprofen filter cake;
[0044] (7) Grind the above filter cake into powder, dry in vacuum at 40°C for 3 hours and weigh to obtain the final product of ibuprofen.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com