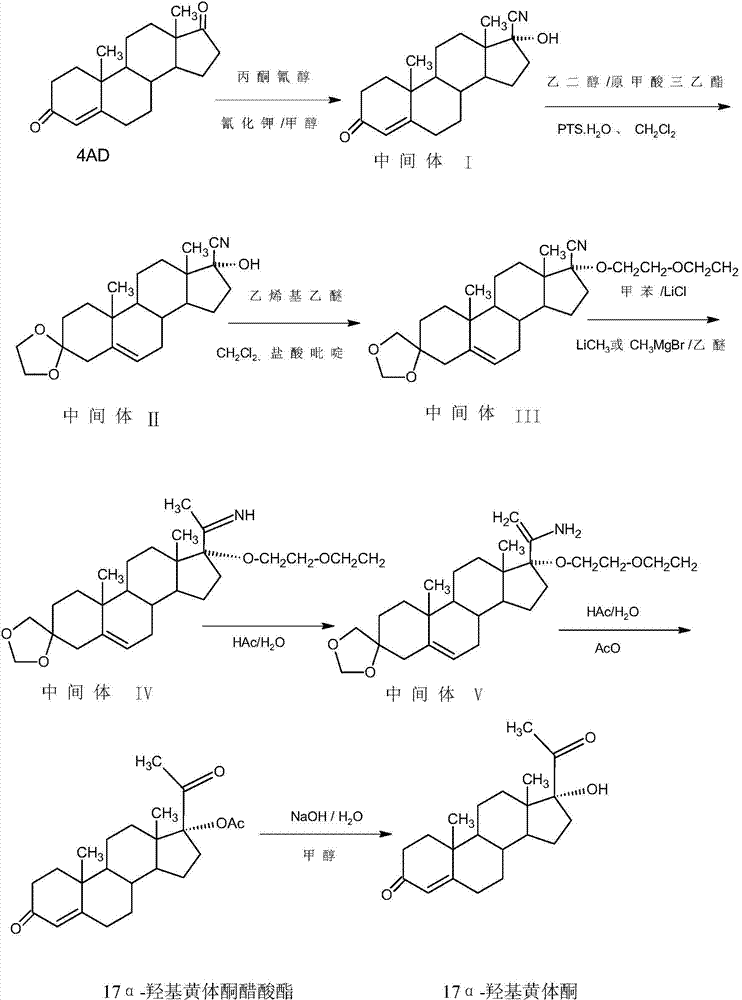
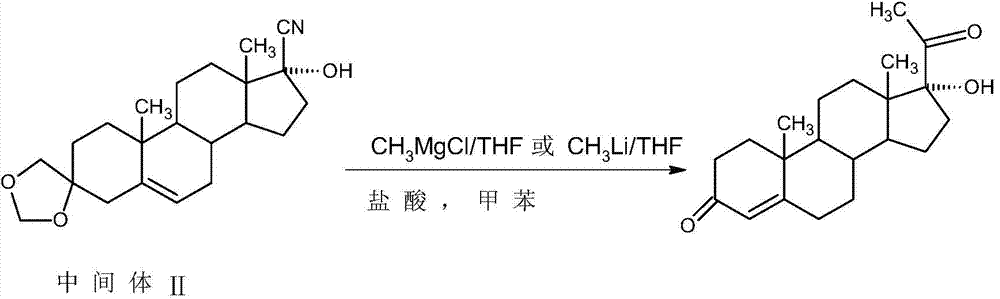
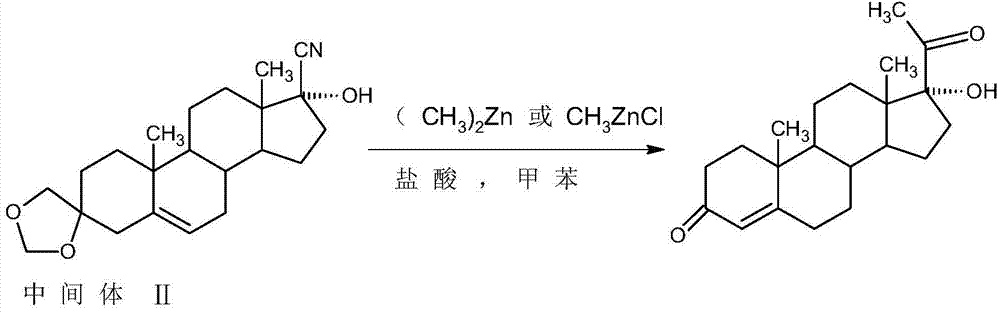
Method for preparing 17alpha-hydroxyprogesteron
A technology of hydroxyprogesterone and cyano, which is applied in the field of preparation of steroid hormone drug intermediates, can solve the problems of large amount of mixed solvents, poor product quality, and many side reactions, and achieve industrial production, production cost reduction, The effect of improving the purity of the crude product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 1000ml three-neck flask, add 50g of intermediate (II) and 200ml of toluene, stir and dissolve at room temperature, then add 1.0g of lithium chloride, slowly raise the temperature to 60-65°C, add dropwise 300ml of 2M methyl zinc toluene solution, After about 1.5~2 hours to drop, after the drop, reflux reaction for 4~5 hours, TLC to detect the reaction end point, after the reaction, add 100ml of 25% ammonium chloride solution, stir for 20~25 minutes to destroy the excess methyl zinc, and then Cool to 5-10°C, separate the water layer, extract the water layer with 200ml of toluene, and send it to the waste water treatment station, combine the organic layer and extract, distill under reduced pressure, recover about 90-95% of toluene, add 400 ml of ethanol, stir and raise the temperature To 55~60°C, add 50ml of 2M hydrochloric acid, continue to heat and stir for 1.5~2 hours, TLC confirms that the reaction is complete, after the reaction, add 10g of sodium bicarbonate to m...
Embodiment 2
[0022] In a 1000ml three-neck flask, add 50g of intermediate (II) and 200ml of toluene, stir and dissolve at room temperature, then add 1.0g of lithium chloride, slowly raise the temperature to 60-65°C, add dropwise 300ml of 2M methyl zinc chloride Toluene solution, about 1.5 to 2 hours to drop, after the drop, reflux reaction for 4 to 5 hours, TLC to detect the reaction end point, after the reaction, add 100ml of 25% ammonium chloride solution, stir for 20-25 minutes to destroy the excess formazan Zinc chloride, then cooled to 5-10°C, separated the water layer, extracted the water layer with 200ml of toluene, then sent to the waste water treatment station, combined the organic layer and extract, distilled under reduced pressure, recovered about 90-95% of toluene, added Add 400 ml of ethanol, stir and heat up to 55-60°C, add 50ml of 2M hydrochloric acid, continue to keep warm and stir for 1.5-2 hours, TLC confirms that the reaction is complete, after the reaction, add 10g of so...
Embodiment 3
[0025] In a 1000ml three-neck flask, add 50g of intermediate (II) and 200ml of tetrahydrofuran, stir and dissolve at room temperature, then add 1.0g of lithium chloride, slowly raise the temperature to 60-65°C, add dropwise 300ml of 2M methyl zinc tetrahydrofuran solution, After about 1.5 to 2 hours of dripping, after the dripping, reflux reaction for 4 to 5 hours, TLC to detect the reaction end point, after the reaction, add 100ml of 25% ammonium chloride solution, stir for 20-25 minutes to destroy the excess methyl zinc, Then distill under reduced pressure to recover about 90-95% of tetrahydrofuran, add 400 ml of ethanol, stir and heat up to 55-60°C, add 75ml of 2M hydrochloric acid, continue to keep warm and stir for 1.5-2 hours, TLC confirms that the reaction is complete, after the reaction , add 12g of sodium bicarbonate to make the pH value 4-4.5, concentrate at normal pressure, recover about 320ml of ethanol, add 400ml of water, continue to distill 100ml of solvent, then...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com