Crystalline Ilaprazole sodium ethylate and preparation method thereof
A technology of ilaprazole sodium and ethanolate, applied in the field of medicine, achieves the effects of good reproducibility, less impurities and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Dissolve solid NaOH in ethanol to form a solution with a pH of about 11. Add the solution to dichloromethane to adjust the alkalinity to about pH 10. The volume ratio of dichloromethane to ethanol is 10:1. 100 mg of ilaprazole sodium was dissolved in the solution to prepare a saturated solution, filtered, and placed in a fume hood to evaporate at room temperature. One day later, 85 mg of light yellow needle-shaped single crystals were obtained, with a yield of 85%.
Embodiment 2
[0034] Dissolve solid NaOH in ethanol to form a solution with a pH of about 11. Add the solution to dichloromethane to adjust the alkalinity to about pH 10. The volume ratio of dichloromethane to ethanol is 10:1. At room temperature, 100 mg of ilaprazole sodium was dissolved in the solution to prepare a saturated solution, filtered, and the solution was drained by a vacuum pump to obtain 90 mg of white powder with a yield of 90%.
Embodiment 3
[0036] In this example, the crystalline ilaprazole sodium ethanolate prepared in Example 1 was measured and characterized, as follows.
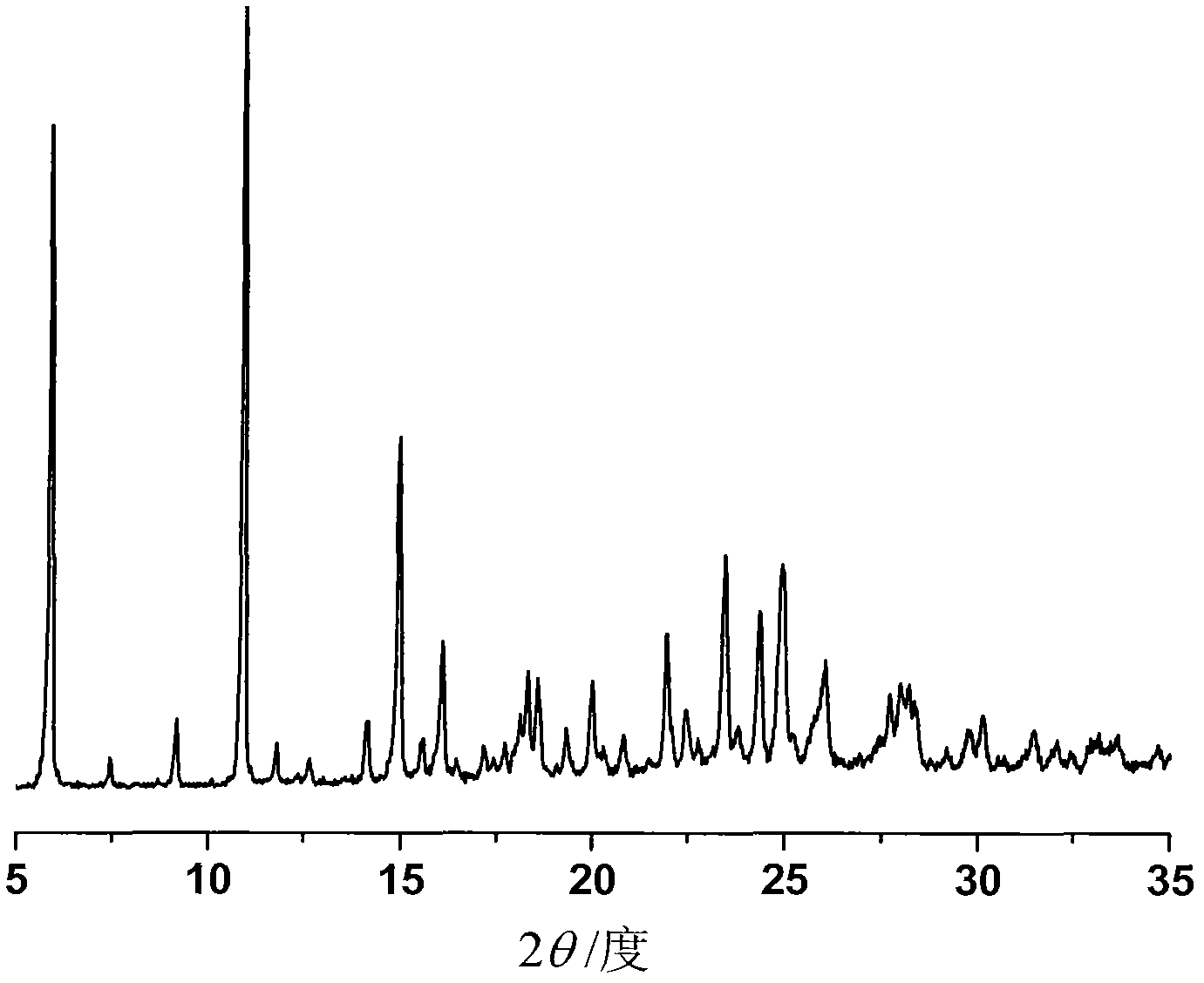
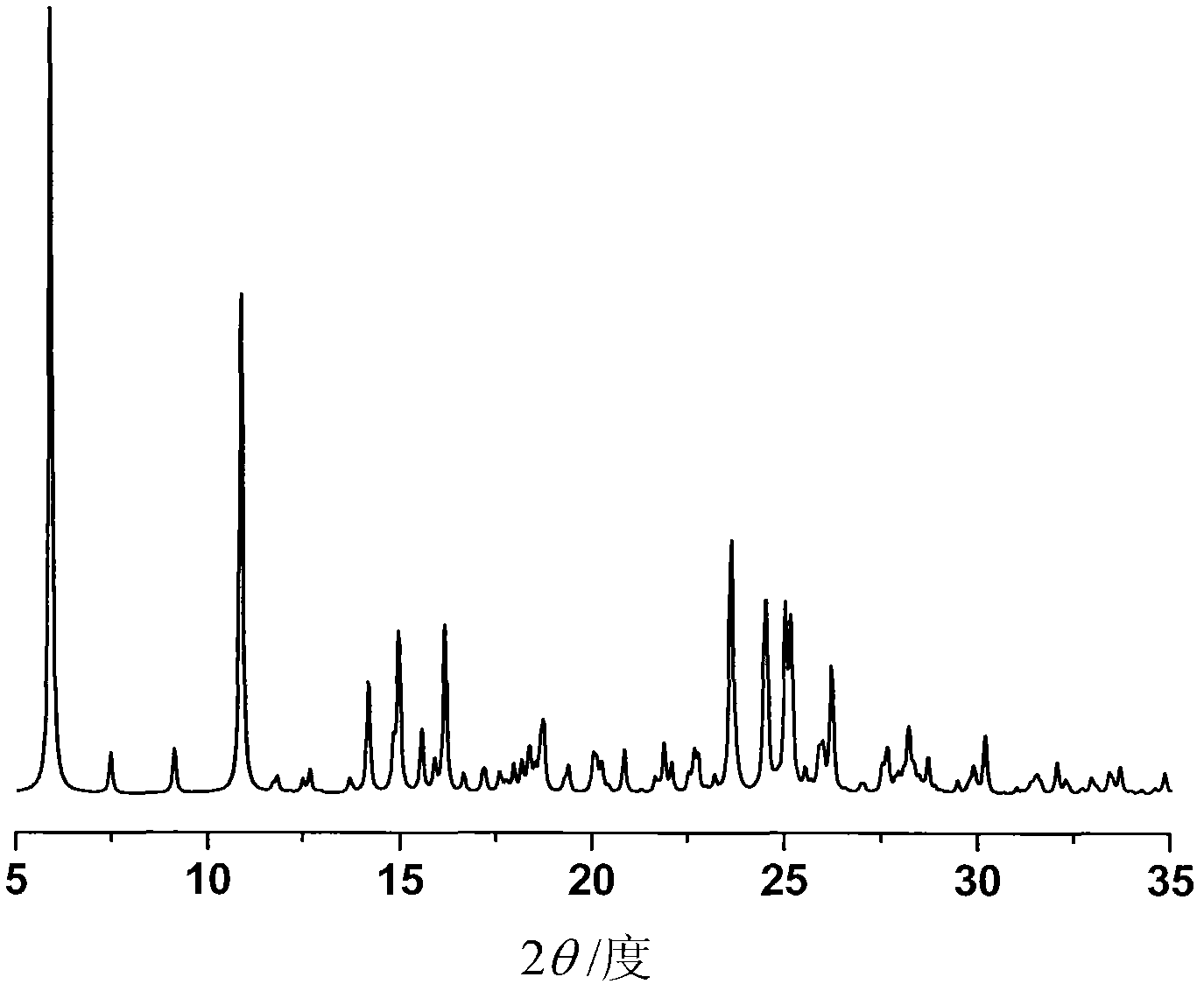
[0037] Adopt Bruker D8 Advance diffractometer to measure the powder diffraction pattern of the crystalline ilaprazole sodium ethanolate that obtains in embodiment 1, measurement condition is as follows: Cu K α, 40kV, 40mV is light source, step-size 0.018 °, scanning speed 4 ° / min, scanning range 5-35°, room temperature, in its powder X-ray diffraction pattern, there are diffraction peaks at the following 2θ diffraction angles: 5.914, 7.479, 9.143, 10.89, 10.902, 14.185, 14.97, 14.991, 15.574, 16.174 , 18.674, 18.754, 20.851, 21.88, 22.668, 23.593, 23.602, 23.646, 23.654, 24.478, 24.53, 24.552, 25.024, 25.034, 25.179, 26.01, 26.222, 28.282, 30. figure 1 shown. Expressed in terms of interplanar distance d, Bragg angle (2θ) and percentage I of relative intensity, as follows:
[0038]
[0039]
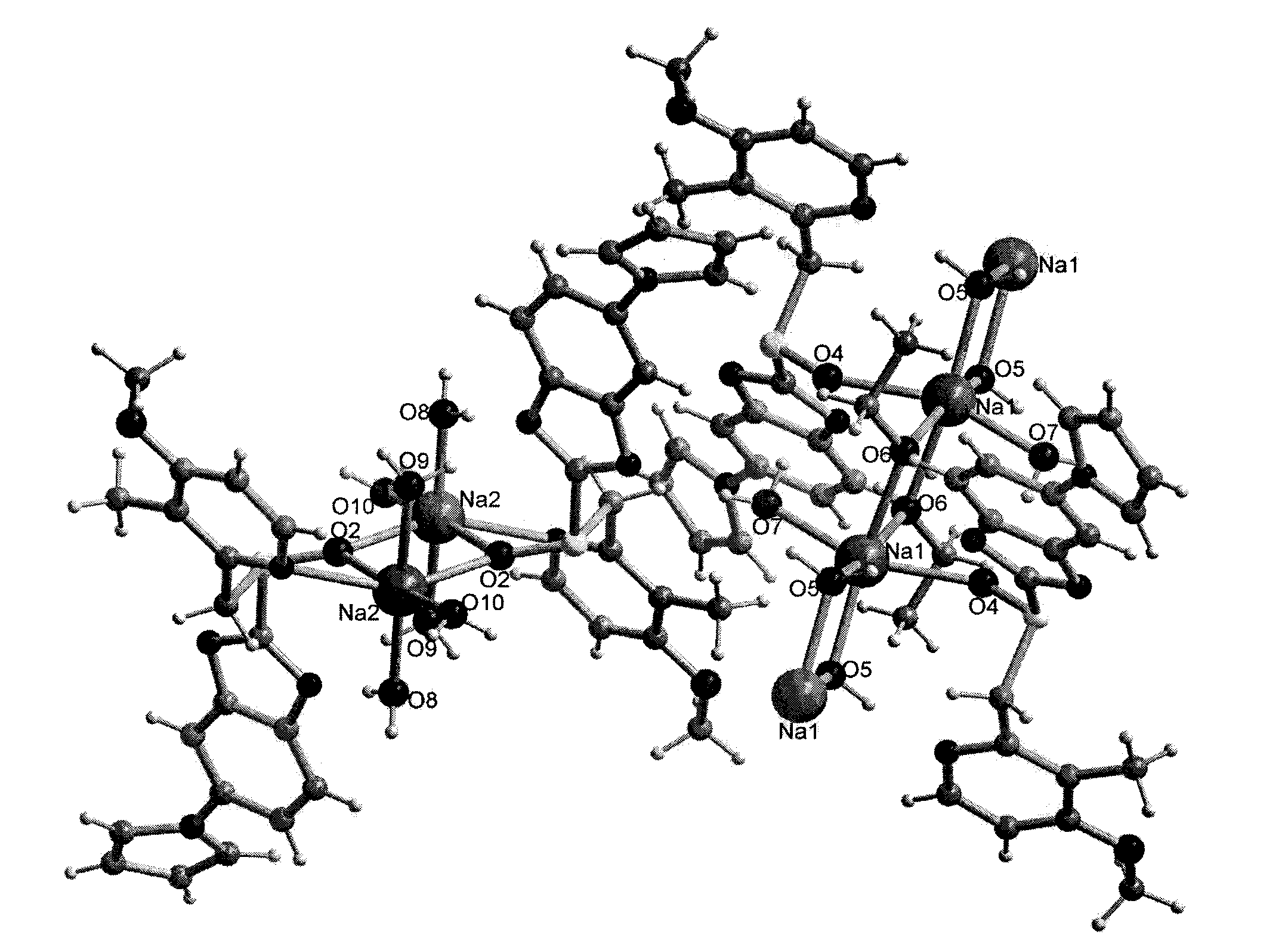
[0040] The crystal structure of the crysta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com