Preparation method of ilaprazole
A technology of ilaprazole and mercaptobenzimidazole, which is applied in the field of preparation of ilaprazole, can solve the problems of high production cost, complicated operation, and many impurities, and achieve the reduction of production power cost, mild reaction conditions, and optimized reaction The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
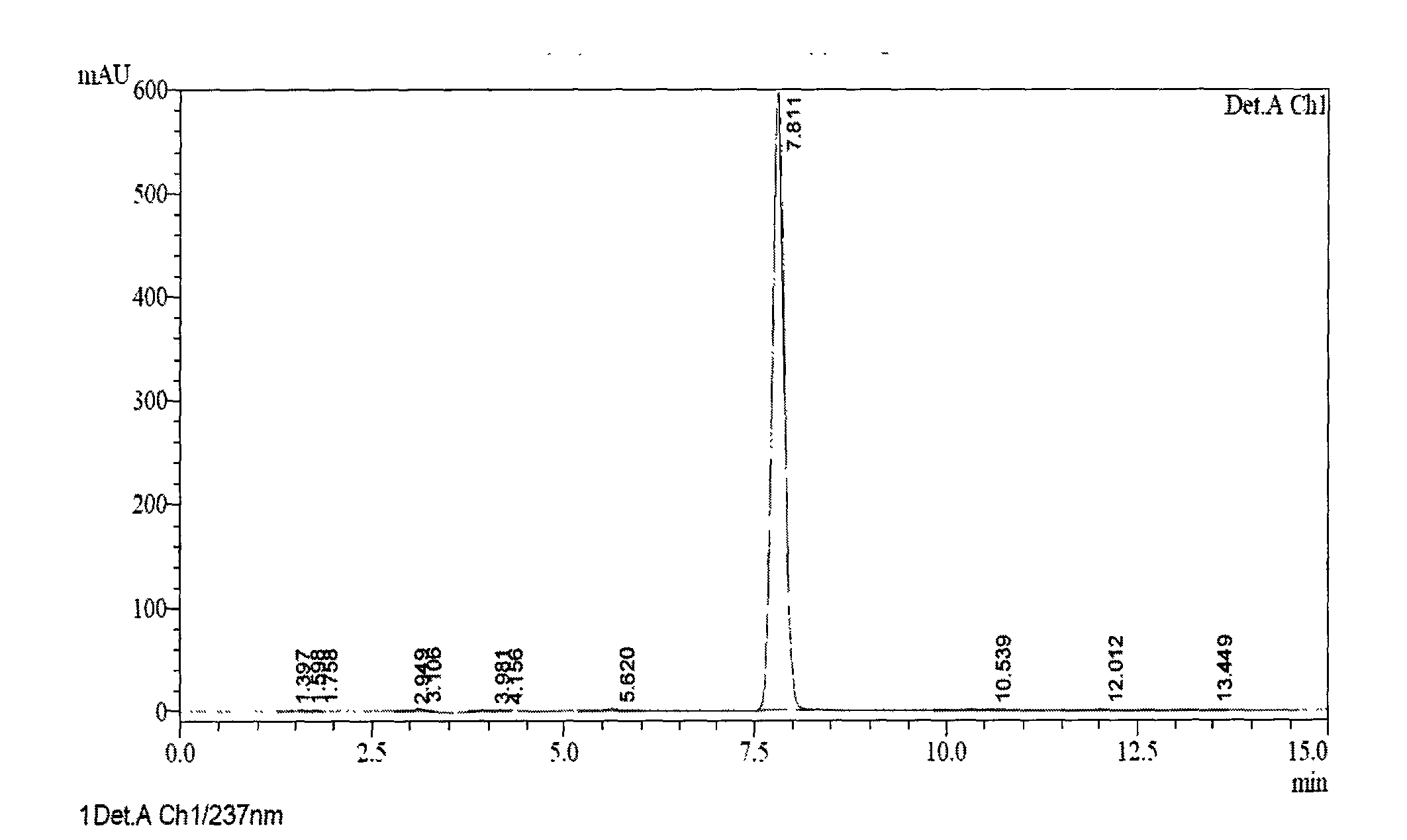
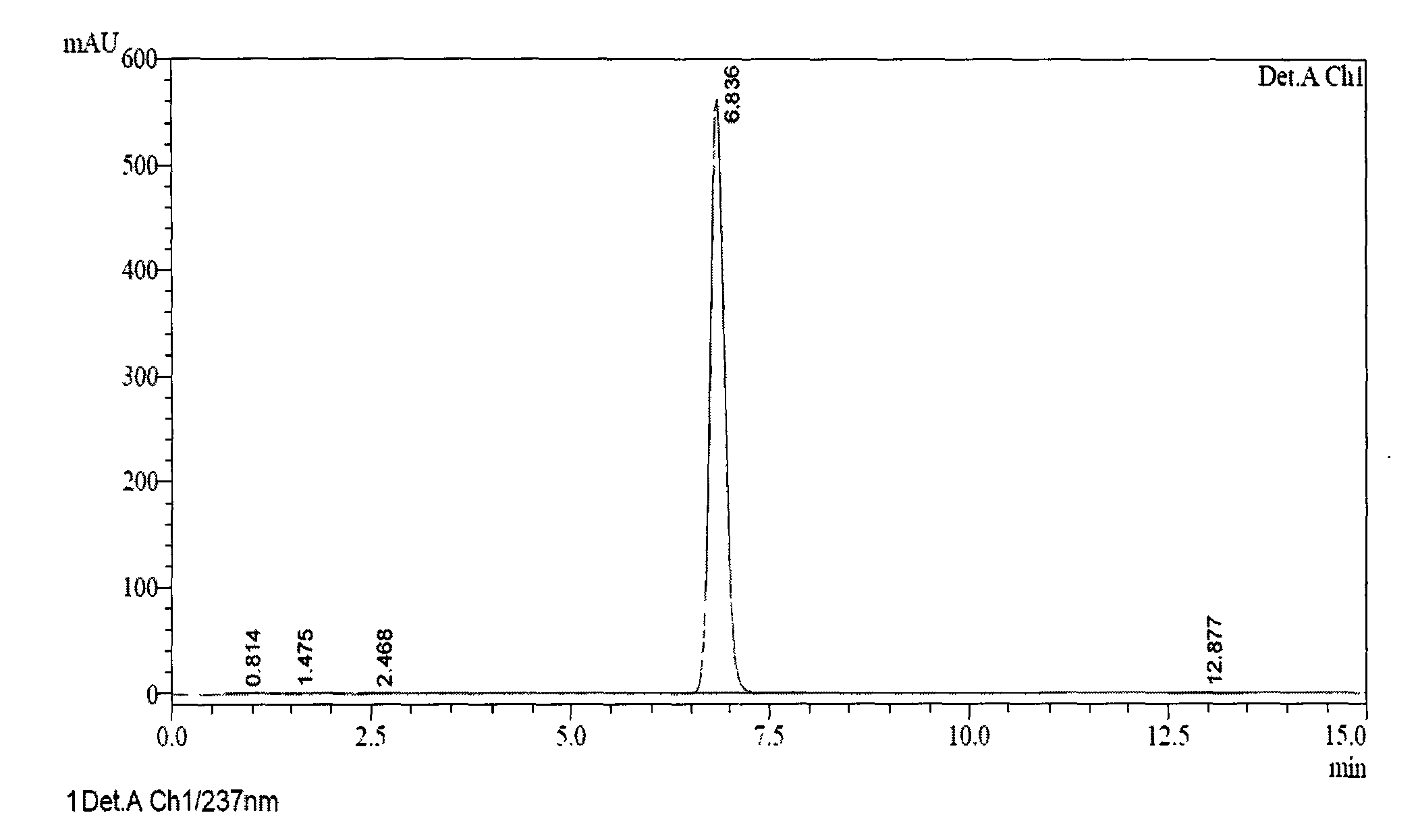
Image
Examples
Embodiment 1
[0054] Embodiment 1 prepares ilaprazole intermediate thioether
[0055] Experiment A
[0056] Operate with reference to Example 1 of Chinese Patent CN94191913.7.
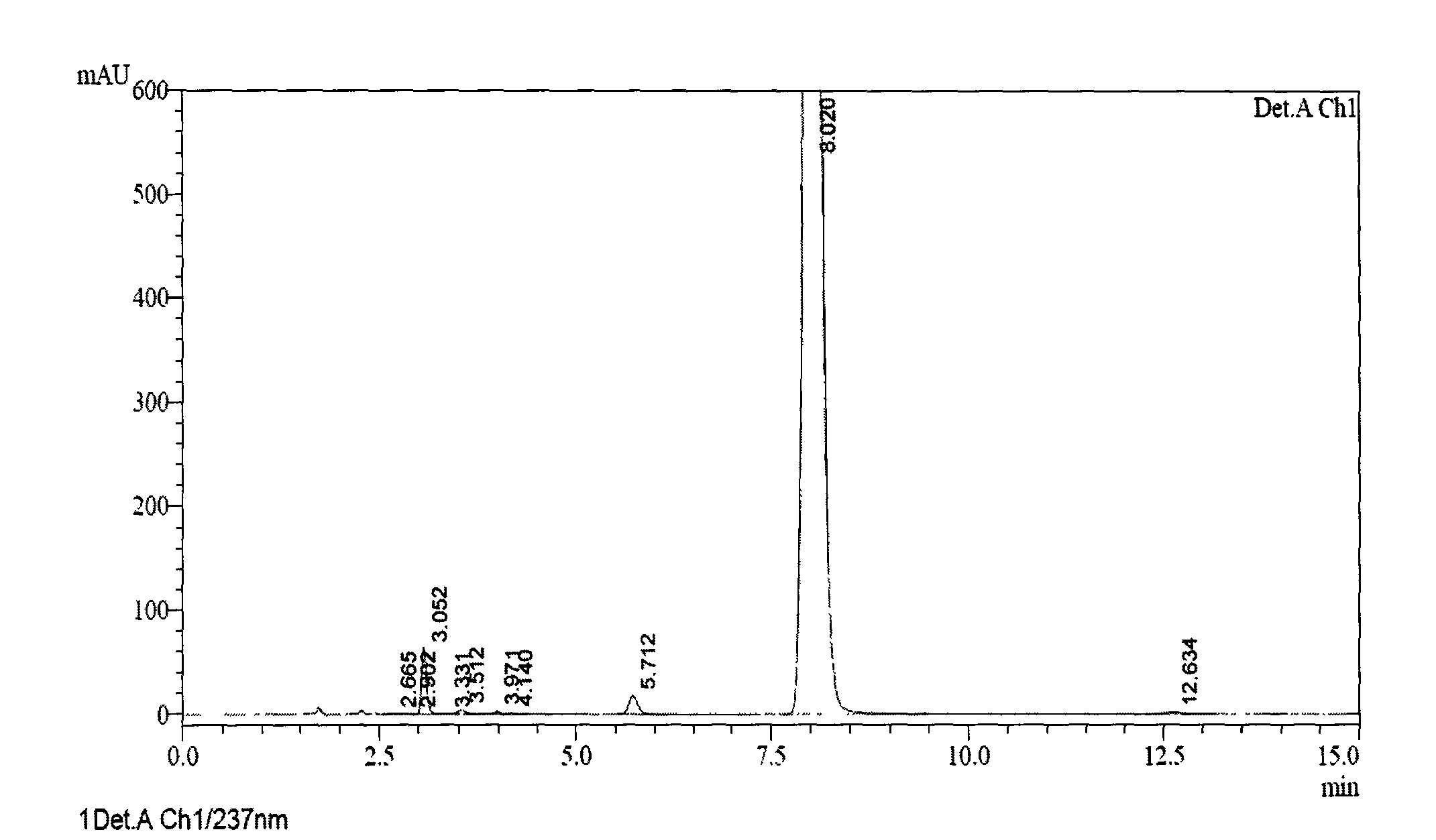
[0057] At room temperature, 2g of 5-(1H-pyrrol-1-yl)-2-mercaptobenzimidazole was dissolved in 0.74g of sodium hydroxide and 100ml of methanol solution, and 3-methyl-4- Methoxy-2-chloromethylpyridine hydrochloride 1.9g, then reacted at 50-60°C for 3 hours, and filtered to remove inorganic substances. The solvent was evaporated under reduced pressure and crystallized from diethyl ether. A light yellow solid was obtained, which was dried to obtain the thioether intermediate of ilaprazole. The yield is 80.5%, and the purity is 98.0% (see accompanying drawing 1).
[0058] The purity detection of the product adopts HPLC method.
[0059] Experiment B
[0060] Operate with reference to Example 22 of Chinese Patent CN200610022315.3.
[0061] At room temperature, 0.66g (2.18mmol) of 5-amino-2[(4-methoxy-3-methyl-2-pyri...
Embodiment 2
[0067] Embodiment 2 prepares ilaprazole intermediate sulfide
[0068] At room temperature, sequentially add 5-(1H-pyrrol-1-yl)-2-mercaptobenzimidazole (85.9g, 0.40mol), sodium hydroxide (31.9g, 0.80mol), organic solvent A (2121ml), ice Stir in a water bath, slowly add 3-methyl-4-methoxy-2-chloromethylpyridine hydrochloride (84.0g, 0.41mol), potassium iodide (3.3g, 0.02mol), slowly heat up to reflux, and react After about 1 hour, the reaction progress was monitored by TLC. After the reaction was complete, part of the solvent was evaporated under reduced pressure, and then purified water was added to obtain a white solid, which was dried to obtain ilaprazole intermediate thioether. (Purity measured by HPLC).
[0069] Organic solvent A
[0070] As can be seen from the above table, the selection of the organic solvent in this step has a great impact on the yield and purity of ilaprazole intermediate thioether, wherein acetone is the optimal choice.
Embodiment 3
[0071] Embodiment 3 prepares ilaprazole intermediate thioether
[0072] At room temperature, 5-(1H-pyrrol-1-yl)-2-mercaptobenzimidazole (85.9g, 0.40mol), inorganic base (31.9g, 0.80mol), acetone (2121ml) were added successively, stirred in an ice-water bath, Slowly add 3-methyl-4-methoxy-2-chloromethylpyridine hydrochloride (84.0g, 0.41mol), potassium iodide (3.3g, 0.02mol), slowly raise the temperature to reflux, and react for about 1h , TLC monitoring of the reaction process, after the reaction is complete, part of the solvent was evaporated under reduced pressure, and then purified water was added to obtain a white solid, which was dried to obtain ilaprazole intermediate thioether. (Purity measured by HPLC).
[0073] Inorganic base
[0075] As can be seen from the above table, the selection of the inorganic base in this step has a great impact on the yield and purity of ilaprazole intermediate sulfide, wherein sodium hydroxi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com