Hydrate of ilaprazole salt, preparation method thereof and application thereof
A compound and water solvent technology, applied in the field of pharmaceutical synthesis, achieves the effects of simple and easy preparation method, obvious therapeutic effect and stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
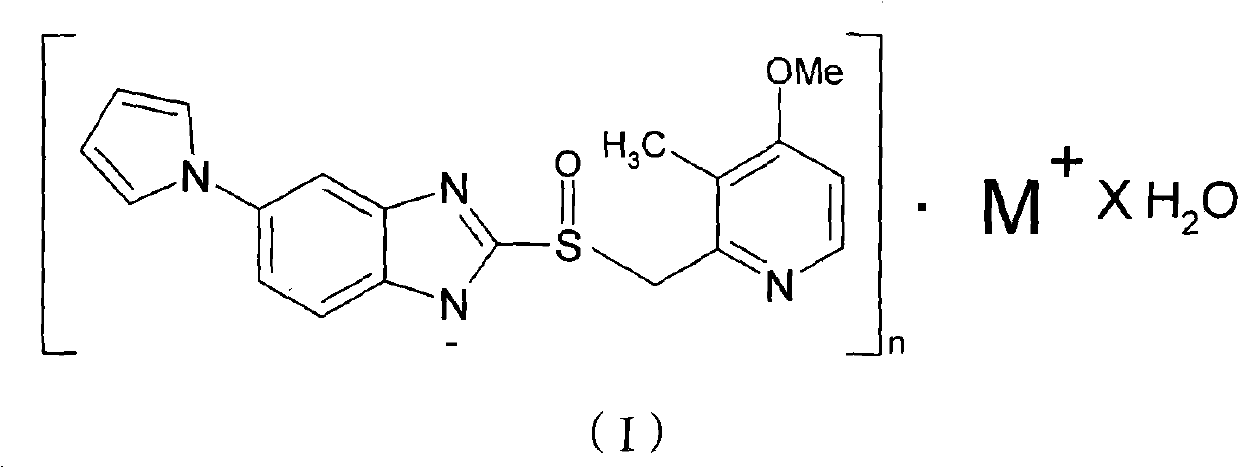
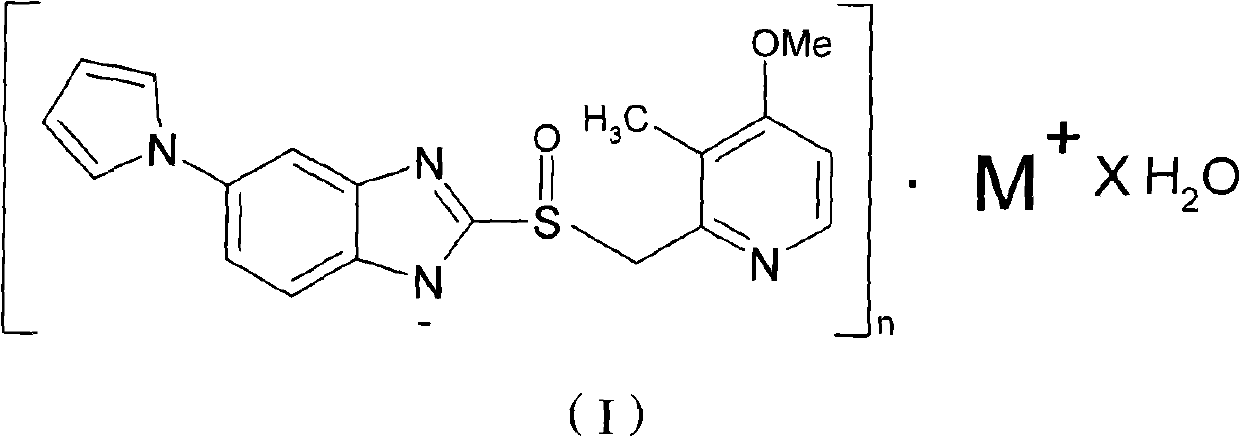
[0029] Synthesis of Ilaprazole Sodium
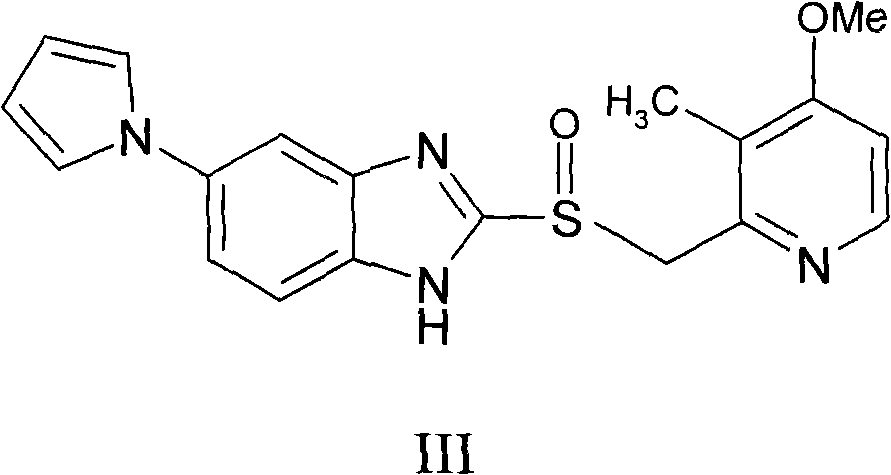
[0030] Add 10.0 g (0.0273 mol) of ilaprazole into 50 ml of methanol, heat up to 50° C., add 4.92 g (0.0273 mol) of methanol solution containing 30% (weight) sodium methoxide, stir for 2 hours, cool to 0° C., and filter , the obtained product was dried under reduced pressure at 60° C. to obtain white crystals, i.e. ilaprazole sodium 9.33 g, yield: 88%, content: 99.9%.
[0031] Elemental analysis was performed on the obtained compound: found values: C, 58.63%; H, 4.53%; N, 14.36%; Na, 6.12%; O, 8.21%; S, 8.15%. Theoretical value of ilaprazole sodium: C, 58.75%; H, 4.41%; N, 14.42%; Na, 5.92%; O, 8.24%; S, 8.26%. It can be seen that the ilaprazole sodium compound was prepared by the above method.
Embodiment 2
[0033] Synthesis of Ilaprazole Sodium
[0034] Add 10.0g (0.0273mol) of ilaprazole into 50ml of methanol, heat up to 50°C, add 1.09g (0.0273mol) of sodium hydroxide dissolved in 10ml of methanol, stir for 2 hours, cool to 0°C, and filter to obtain The product was dried under reduced pressure at 60°C to obtain white crystals, i.e. ilaprazole sodium 9.33g, yield: 88%, content: 99.9%.
[0035] Elemental analysis of the obtained compound: found values: C, 58.53%; H, 4.59%; N, 14.35%; Na, 6.05%; O, 8.28%; S, 8.20%. Theoretical value of ilaprazole sodium: C, 58.75%; H, 4.41%; N, 14.42%; Na, 5.92%; O, 8.24%; S, 8.26%. It can be seen that the ilaprazole sodium compound was prepared by the above method.
Embodiment 3
[0037] Synthesis of Ilaprazole Sodium
[0038] Add 10.0 g (0.0273 mol) of ilaprazole into 50 ml of methanol, add 1.09 g (0.0273 mol) of sodium hydroxide, stir at room temperature for 2 hours, concentrate, add n-butanol, and add isopropyl ether to crystallize at room temperature. C and dried under reduced pressure to obtain white crystals, i.e. ilaprazole sodium 9.5g, yield: 90%, content: 99.8%.
[0039] Elemental analysis of the obtained compound: measured value: C, 58.60%; H, 4.57%; N, 14.35%; Na, 6.09%; O, 8.29%; S, 8.10%; C, 58.75%; H, 4.41%; N, 14.42%; Na, 5.92%; O, 8.24%; It can be seen that the ilaprazole sodium compound was prepared by the above method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com