Lacidipine-spirolactone co-amorphous solid dispersion and preparation thereof
A technology of solid dispersion and lacidipine, which is applied in the field of medicine, can solve problems such as limiting oral bioavailability, and achieve the effect of improving in vitro dissolution rate, good economy, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
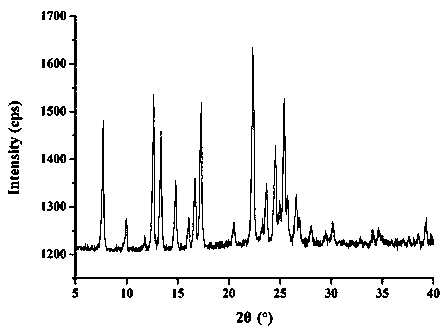
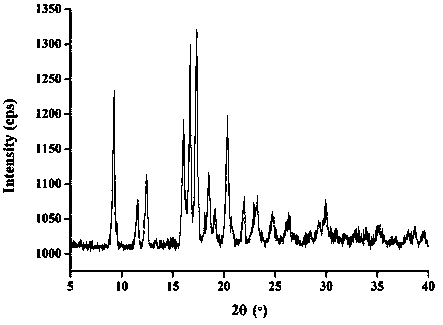
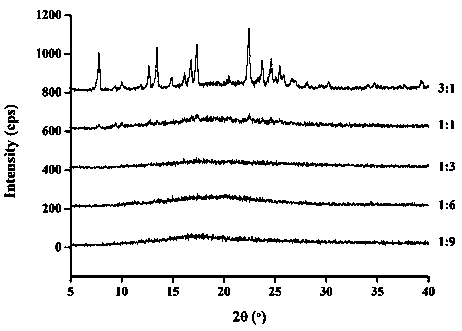
[0068] The molar ratio of lacidipine to spironolactone is 3:1. Weigh 0.5g lacidipine bulk drug and 0.15g spironolactone bulk drug, add appropriate amount of ethanol to dissolve until clarified. Rotate the solvent under reduced pressure at 20-60° C., and dry the residue in vacuum for 24 hours to obtain the obtained product. Measure the dissolution rate of lacidipine according to the dissolution measurement method described below to be 5.1%, and the dissolution rate of spironolactone is: 46.2%.
Embodiment 2
[0070] The molar ratio of lacidipine to spironolactone is 1:1. Weigh 0.5g lacidipine bulk drug and 0.46g spironolactone bulk drug, add appropriate amount of ethanol to dissolve until clarified. The preparation process is the same as in Example 1. Measure the dissolution rate of lacidipine according to the dissolution rate determination method described below to be 21%, and the dissolution rate of spironolactone is: 57%.
Embodiment 3
[0072] The molar ratio of lacidipine to spironolactone is 1:3. Weigh 0.5g lacidipine bulk drug and 1.37g spironolactone bulk drug, add appropriate amount of ethanol to dissolve until clarified. The preparation process is the same as in Example 1. Measure the dissolution rate of medicine according to the dissolution rate determination method described below to be 56%, and the dissolution rate of spironolactone is: 60.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com