Cilnidipine nano-suspension and preparation method thereof
A technology of nanodipine and cilnidipine, applied in the field of medicine, can solve the problems such as no cilnidipine nanosuspension/nanocrystal report, etc., and achieve the effects of improving in vitro dissolution characteristics, preventing aggregation and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of cilnidipine nanosuspension
[0044] Weigh 0.20 g of Poloxamer 407 (P407), add it into 50 mL of distilled water, and stir it magnetically to dissolve; then add 1.00 g of cilnidipine raw material, and stir it magnetically for 15 minutes to obtain a coarse suspension. This coarse suspension is then transferred to a nanomill for grinding. The grinding conditions are as follows: the diameter of the yttrium-stabilized zirconia grinding beads is 0.6-0.8 mm, and the rotation speed of the stirring shaft is 2000 rpm. After 60 minutes, a sample was taken, and the measured particle diameter was 435nm, and the PDI was 0.190.
Embodiment 2
[0045] Embodiment 2: the preparation of cilnidipine nanosuspension
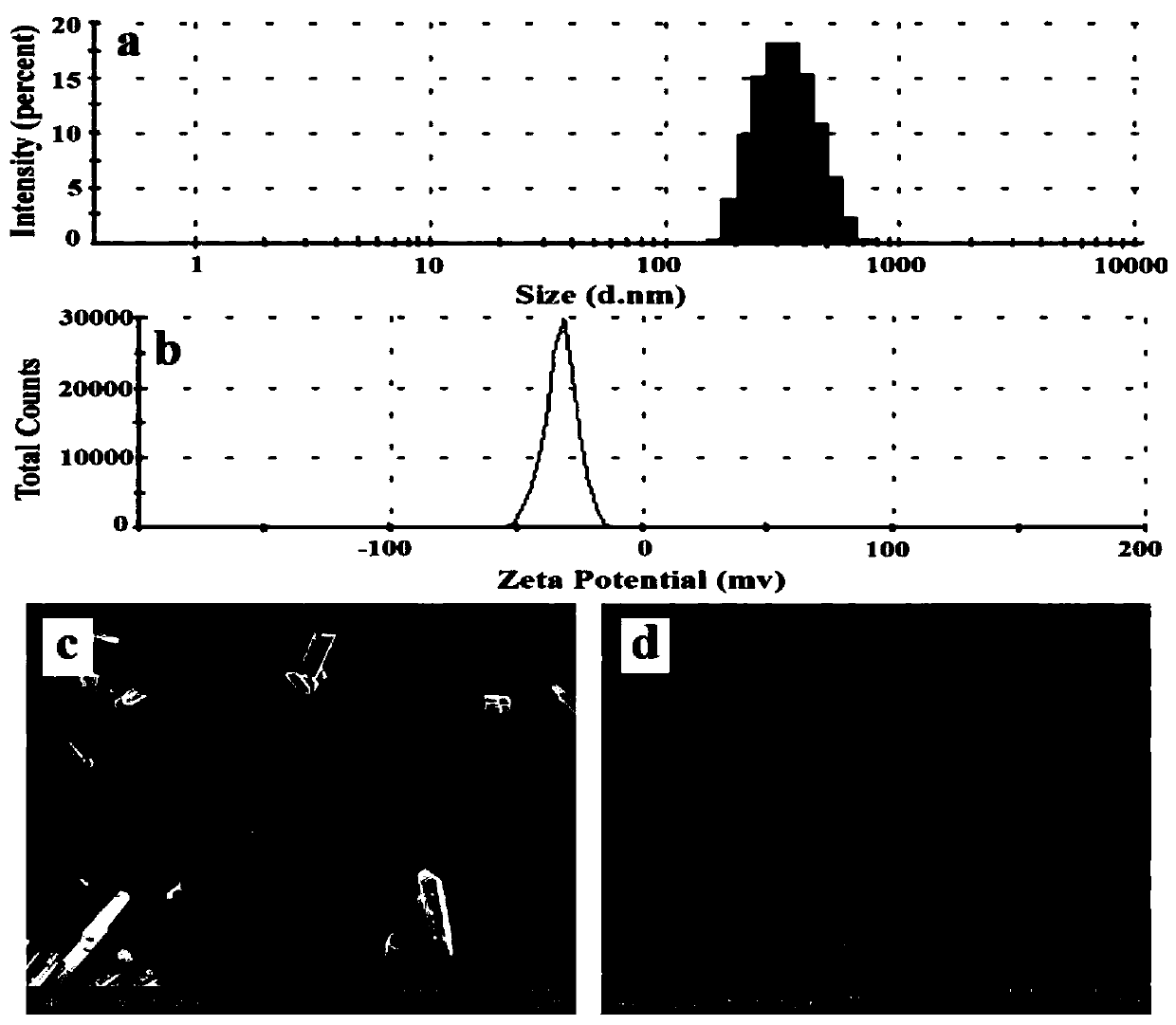
[0046] Weigh 0.05g of sodium lauryl sulfate and 0.30g of polyvinylpyrrolidone-vinyl acetate copolymer (PVP VA64), add them into 50mL of distilled water, stir to dissolve; then add 1.00g of cilnidipine raw material, and stir for 15 minutes , to obtain a coarse suspension. This coarse suspension is then transferred to a nanomill for grinding. The grinding conditions are as follows: the diameter of the yttrium-stabilized zirconia grinding beads is 0.6-0.8 mm, and the rotation speed of the stirring shaft is 2500 rpm. After 45 minutes, the sample was taken, and the measured particle size was 312nm, and the potential was -33.6mV. See the results figure 1 a and figure 1 b.
Embodiment 3
[0047] Embodiment 3: the preparation of cilnidipine nanosuspension
[0048] Weigh 0.10 g of sodium lauryl sulfate and 0.30 g of hydroxypropyl cellulose (HPC-SSL), add it into 50 mL of distilled water, and stir it magnetically to dissolve it, add 1.00 g of cilnidipine raw material, and stir it magnetically for 15 minutes to obtain crude suspension. This coarse suspension is then transferred to a nanomill for grinding. The grinding conditions are as follows: the diameter of the yttrium-stabilized zirconia grinding beads is 0.6-0.8 mm, and the rotation speed of the stirring shaft is 2500 rpm. After 45 minutes, a sample was taken, and the measured particle size was 357nm, and the potential was -32.3mV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com