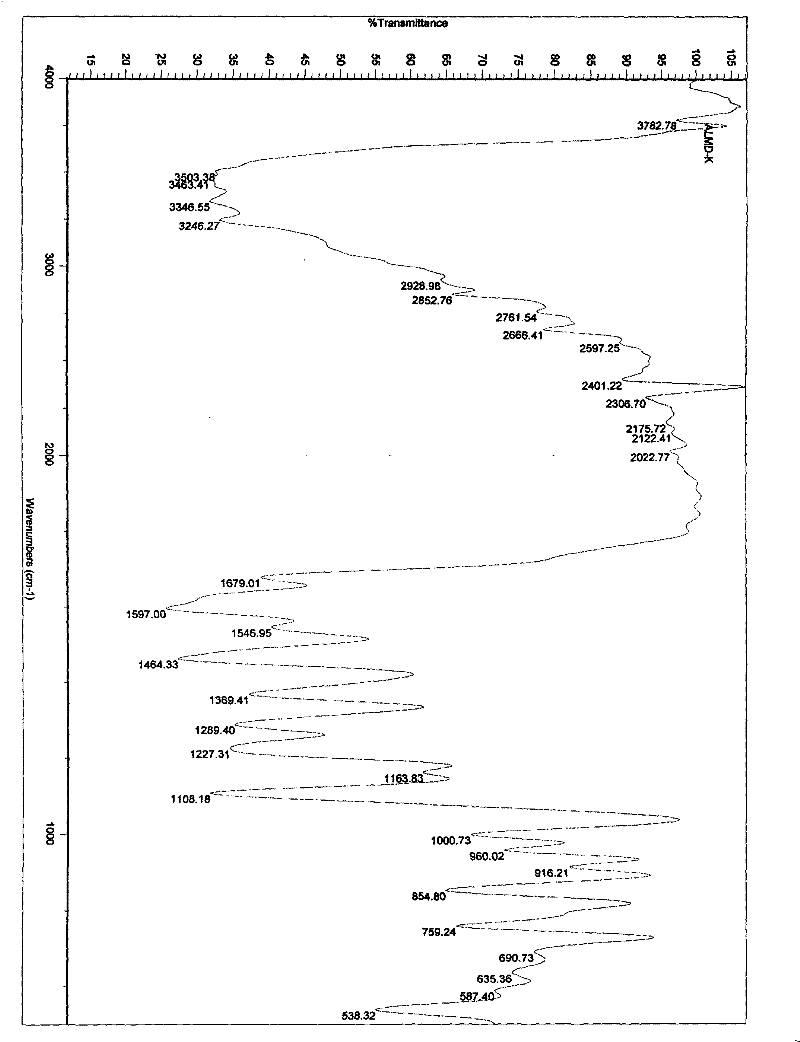
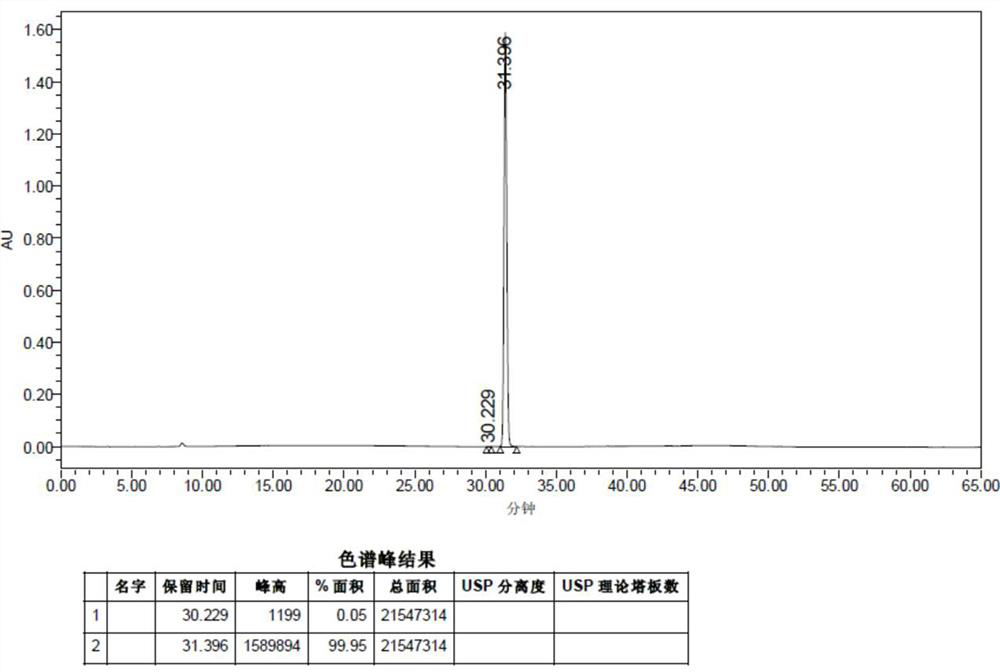
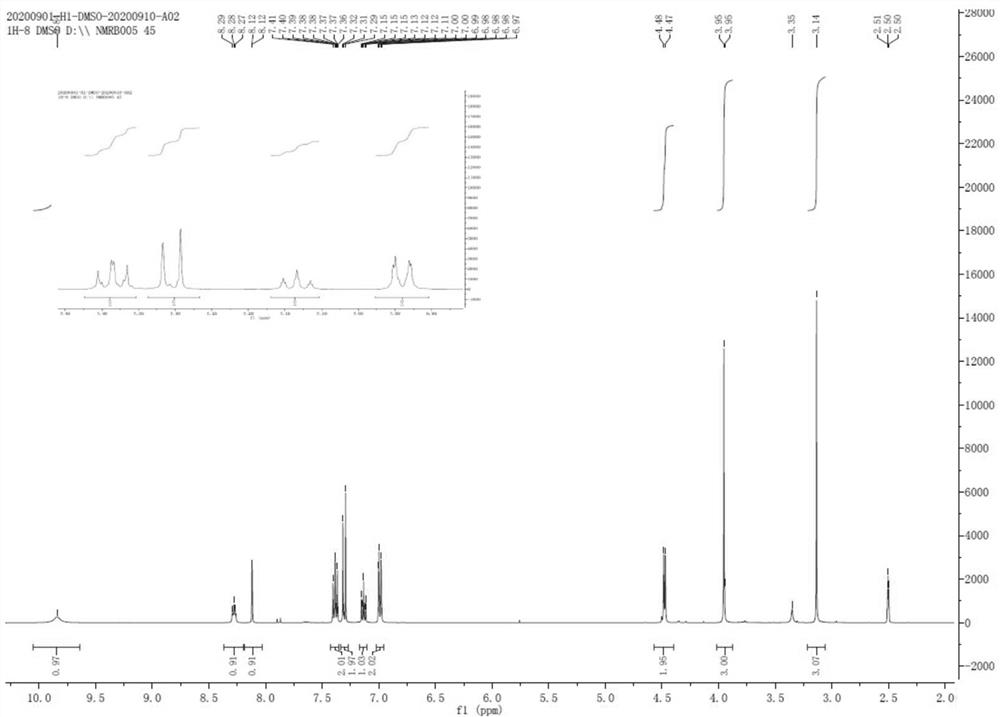
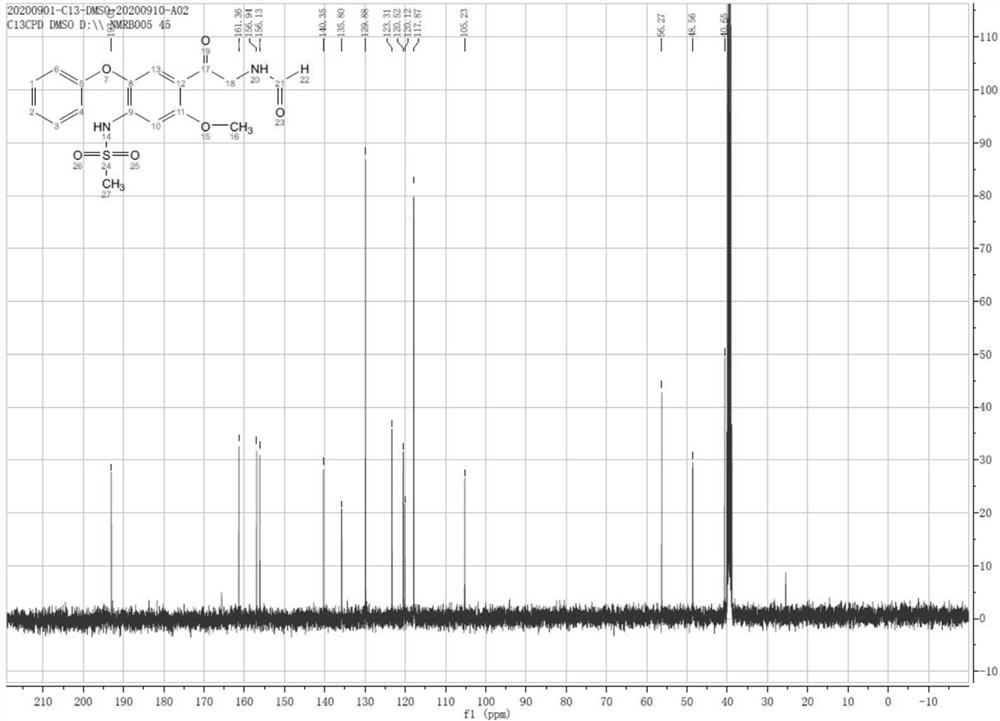
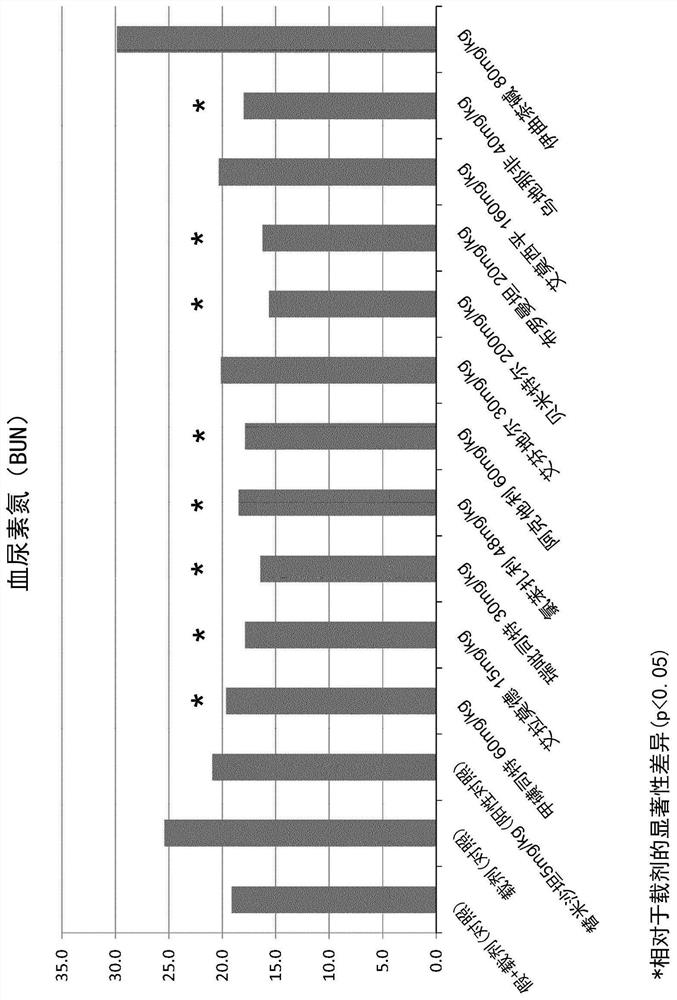
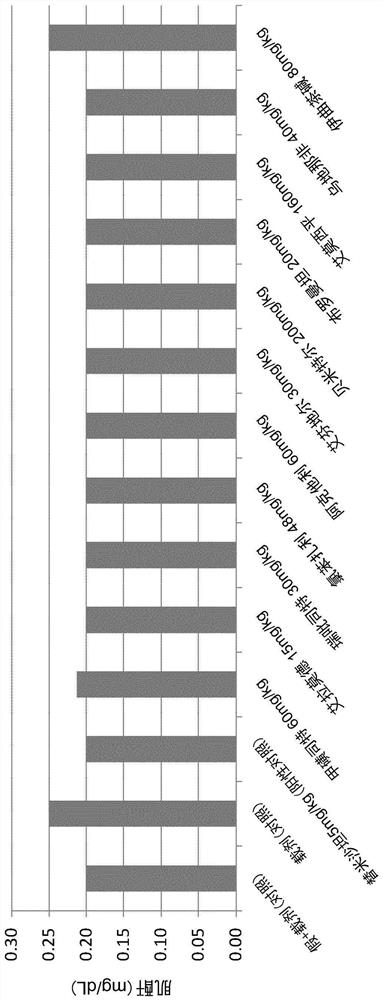
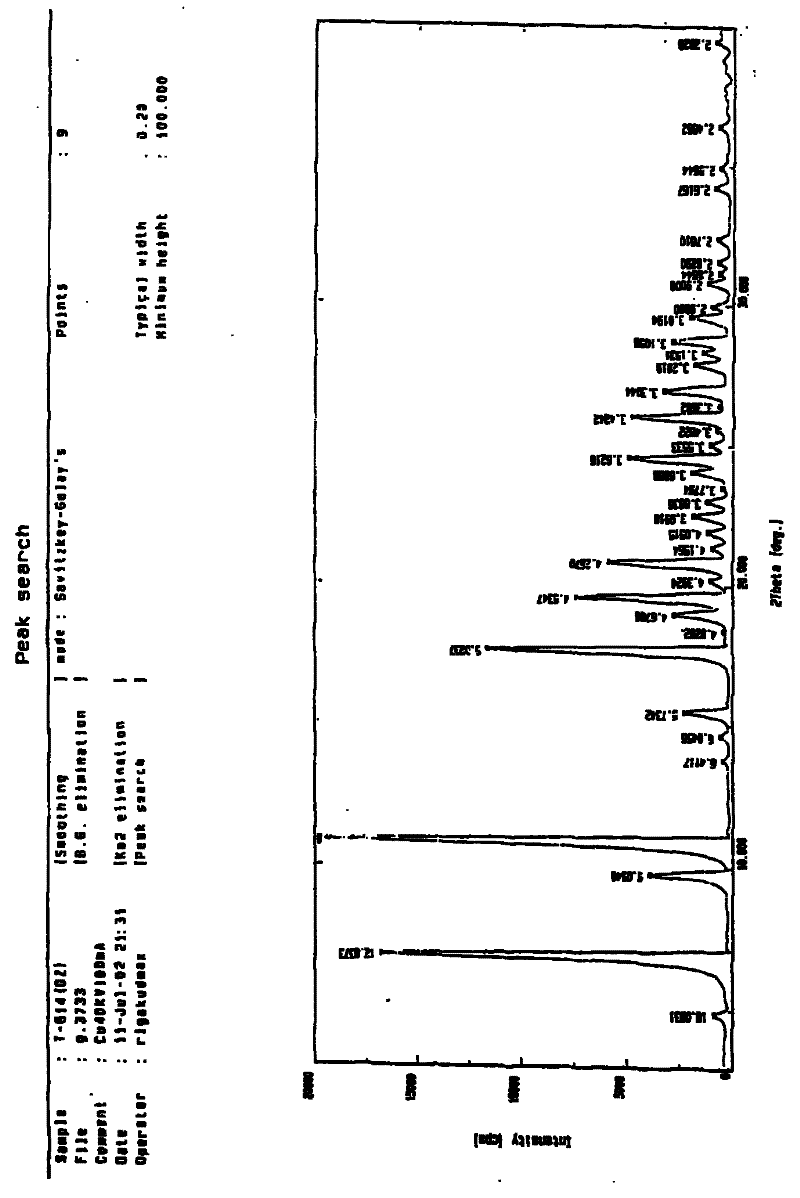
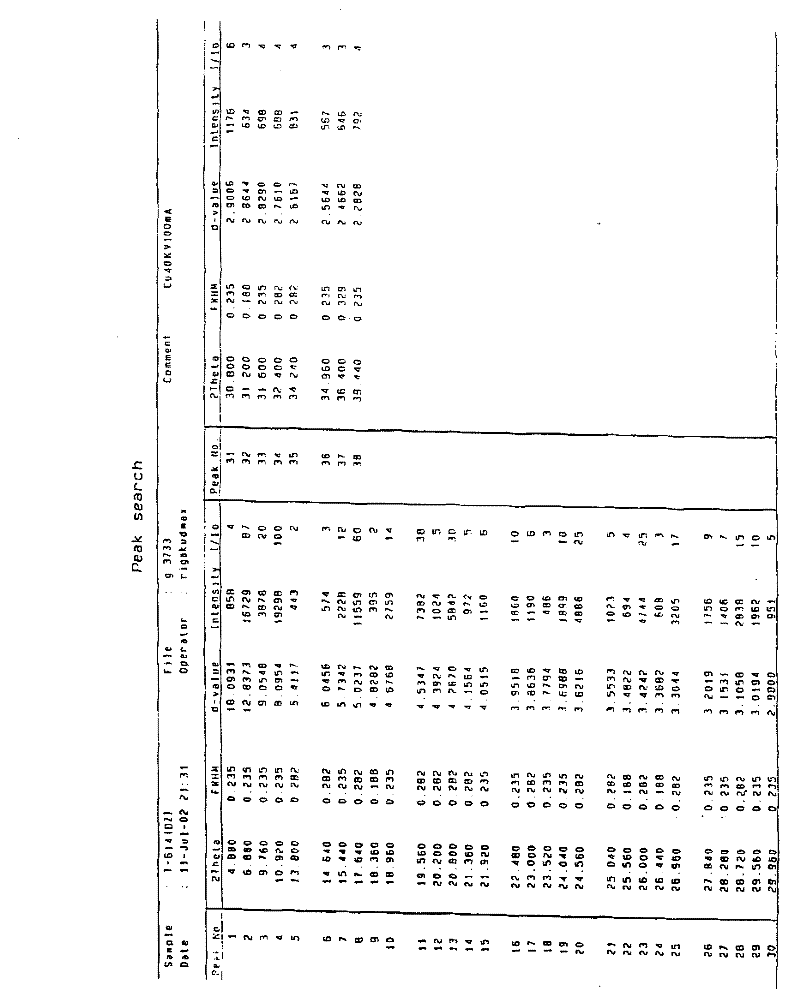
Patents
Literature
32 results about "Iguratimod" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
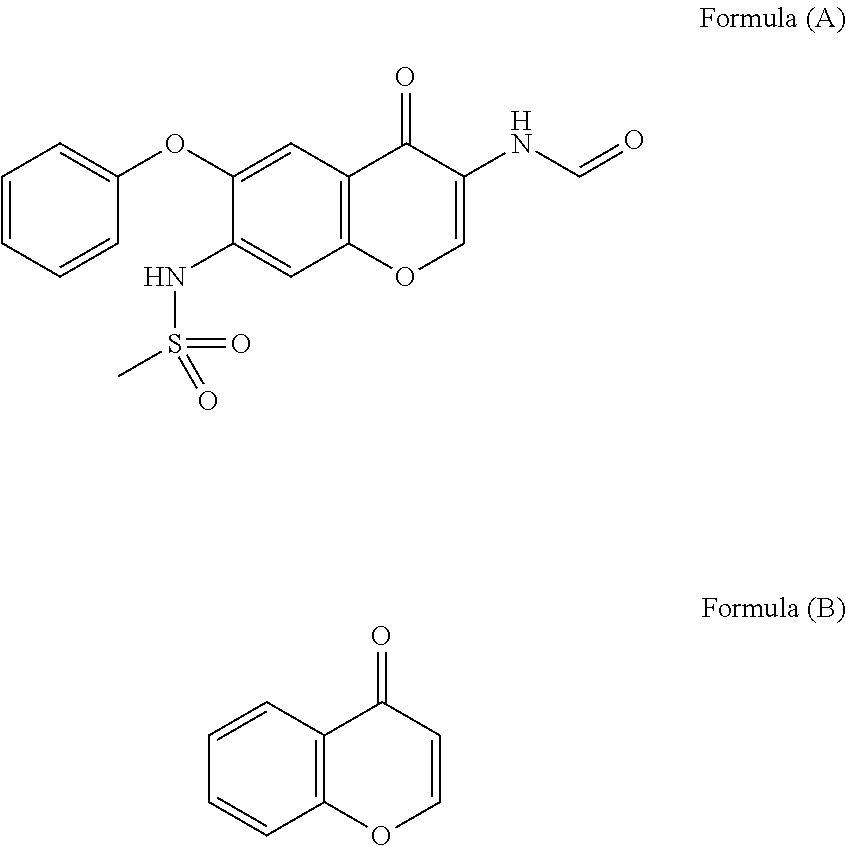
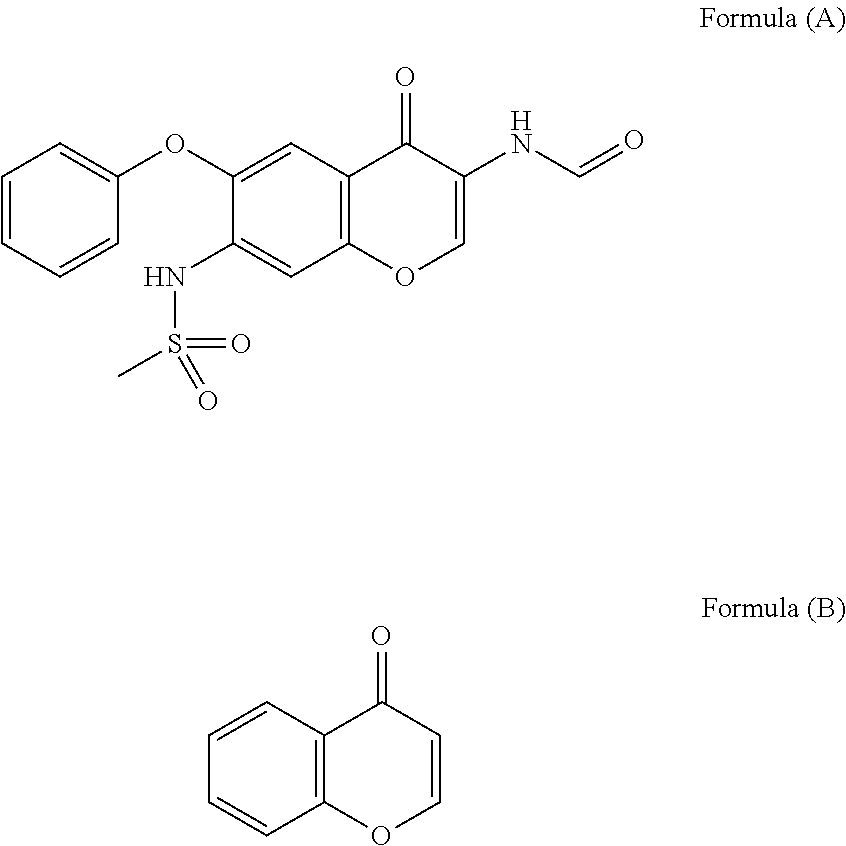
Iguratimod is an anti-inflammatory small molecule drug used for the treatment of rheumatoid arthritis, together with methotrexate in Japan and China. As of 2015 the biological target was not known, but it prevents NF-κB activation and subsequently selectively inhibits COX-2 and several inflammatory cytokines.
Percutaneous absorption agent containing Ailamode, preparation method and medical uses thereof
InactiveCN101401783AAvoid damageAvoid first pass reactionOrganic active ingredientsAntipyreticPercutaneous absorptionIguratimod
The invention relates to a transdermal absorbent containing Iguratimod, which comprises gellies, ointment, and cream, wherein the mass percentage content of the Iguratimod in the transdermal absorbent is between 0.1 and 10 percent, and preferably the mass percentage content of the Iguratimod in the transdermal absorbent is between 0.4 and 3 percent; and the invention also discloses requirements of the transdermal absorbent on the selections of matrix and a transdermal enhancer. Furthermore, the invention also provides a method for preparing the transdermal absorbent containing the Iguratimod and pharmaceutical application thereof.
Owner:杨喜鸿
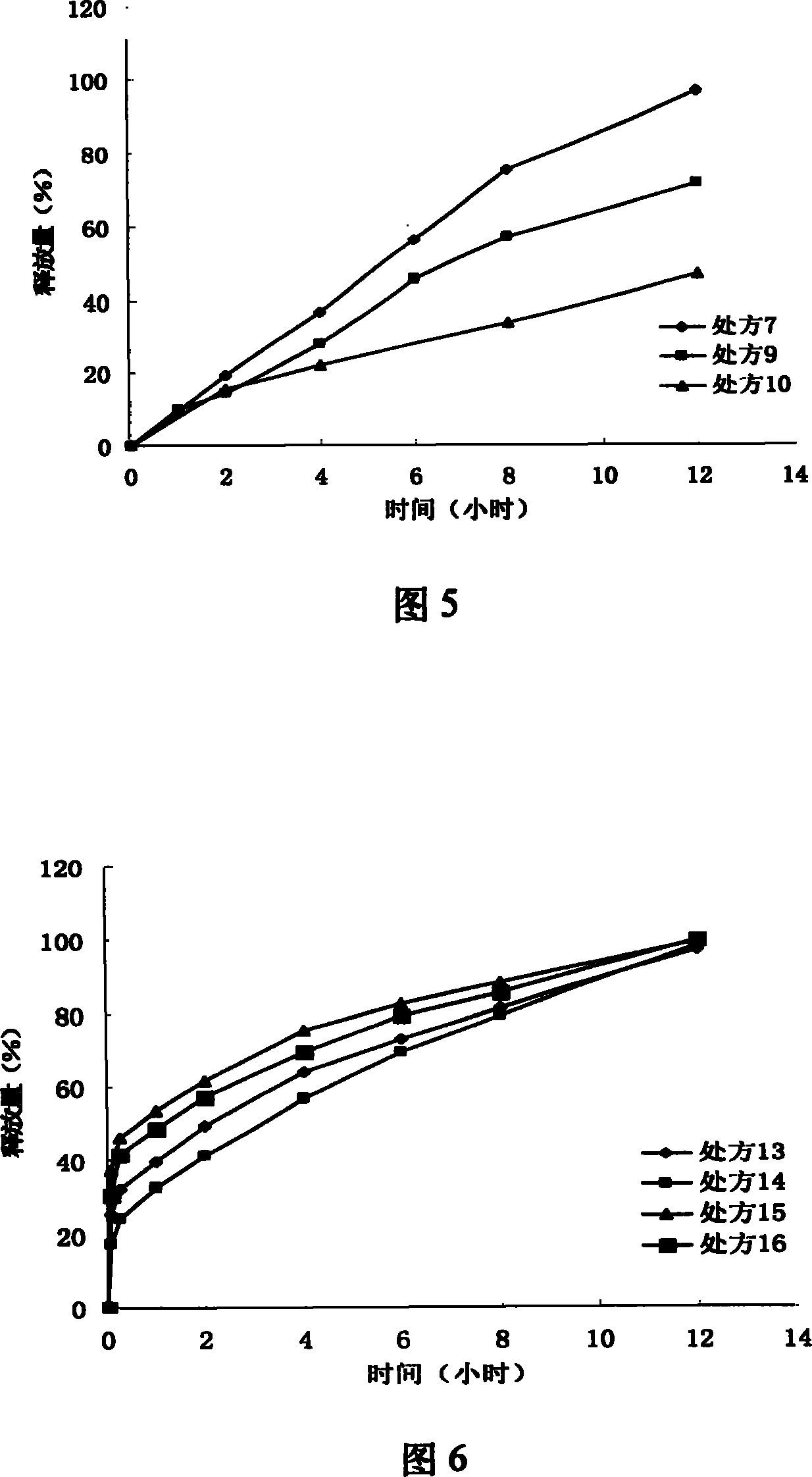
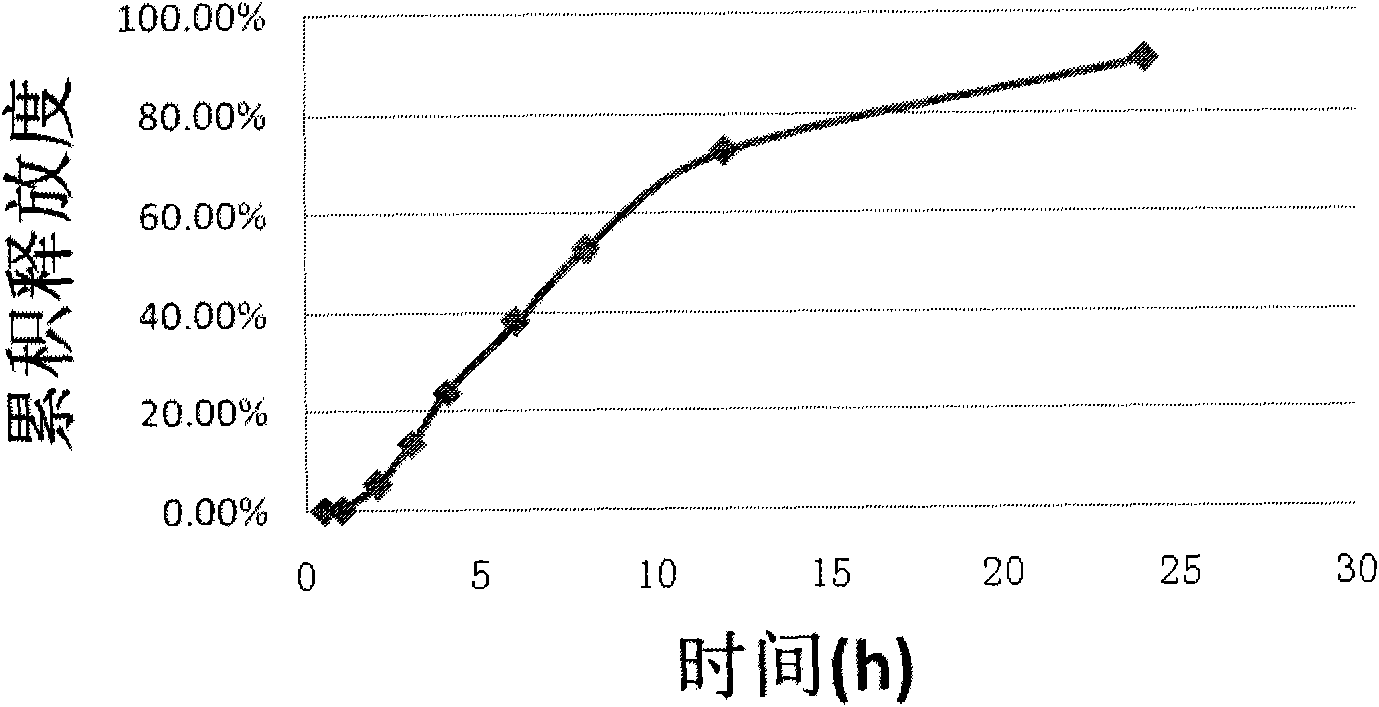
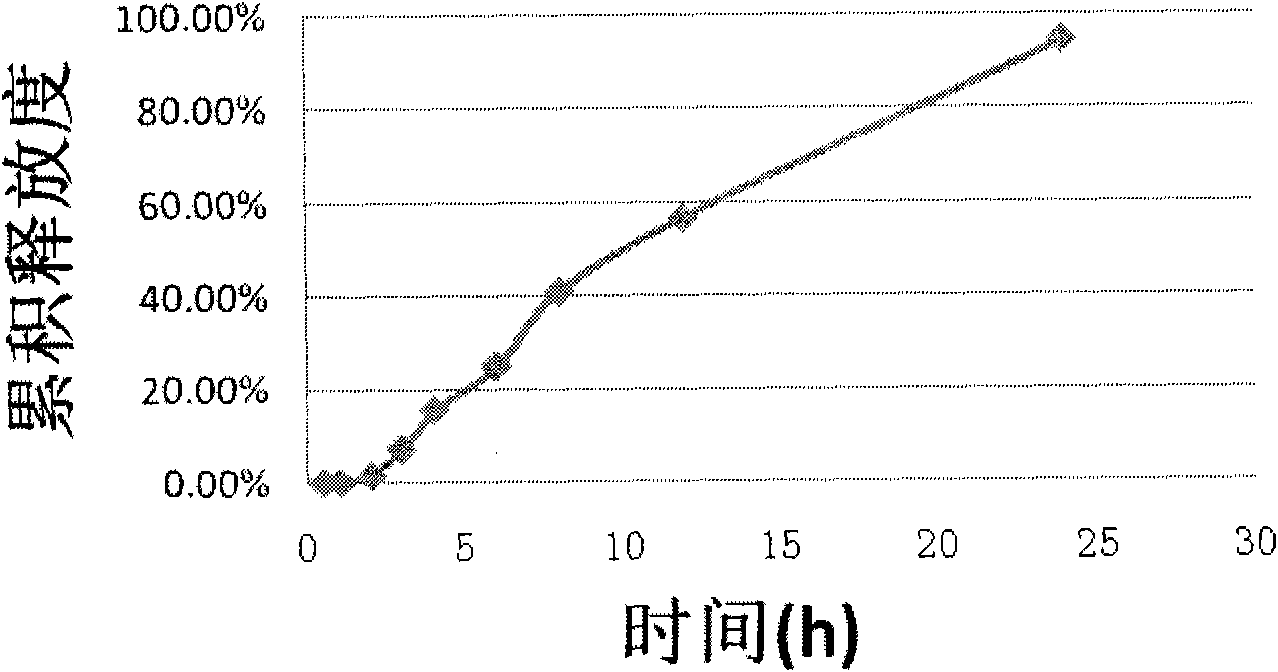
Iguratimod oral double-layer sustained-release preparation
InactiveCN101095671AAccelerate time to peak blood concentrationImprove in vitro dissolutionOrganic active ingredientsAntipyreticSide effectEffective action
The invention relates to oral double iguratimod controlled release formulation, which comprises fast release layer and slow release layer that are composed of 8-30% micronizing iguratimod crystal powder and medical findings, and the granule size of iguratimod crystal powder is 1-10 um. The effective component in fast release layer is released in short time and reaches to effective blood chemical concentration for effective action; the iguratimod in sloe release layer is released gradually and maintains effective blood medical concentration for continuous effective action. The invention overcomes shortcomings of short effective action time and a little high toxic effect.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
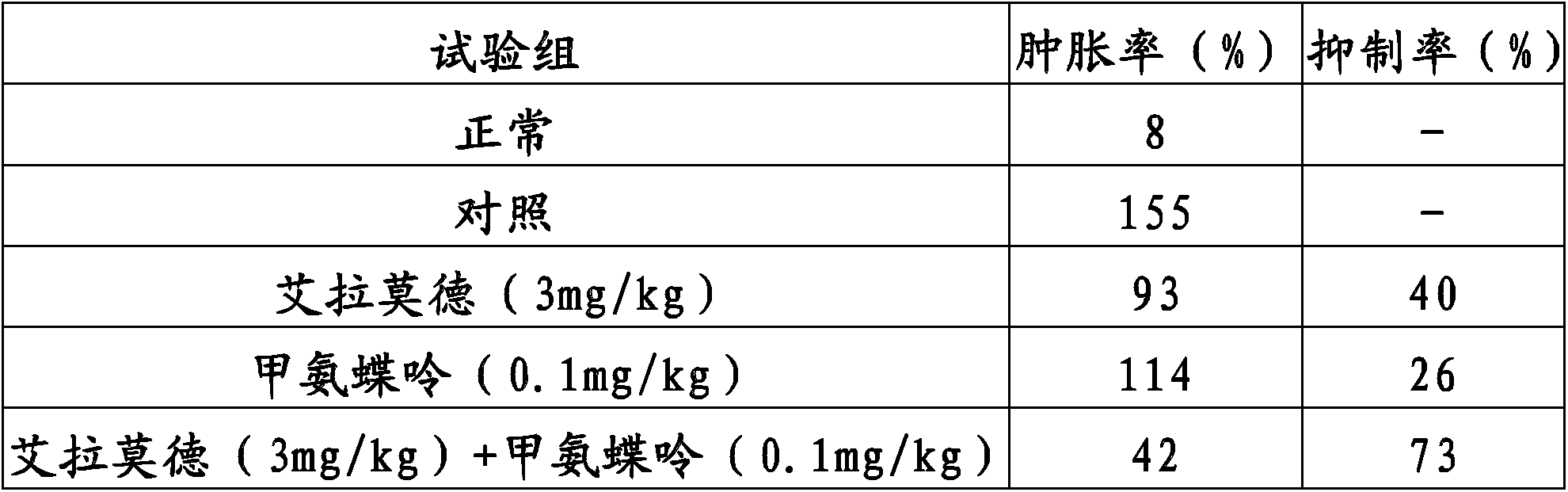
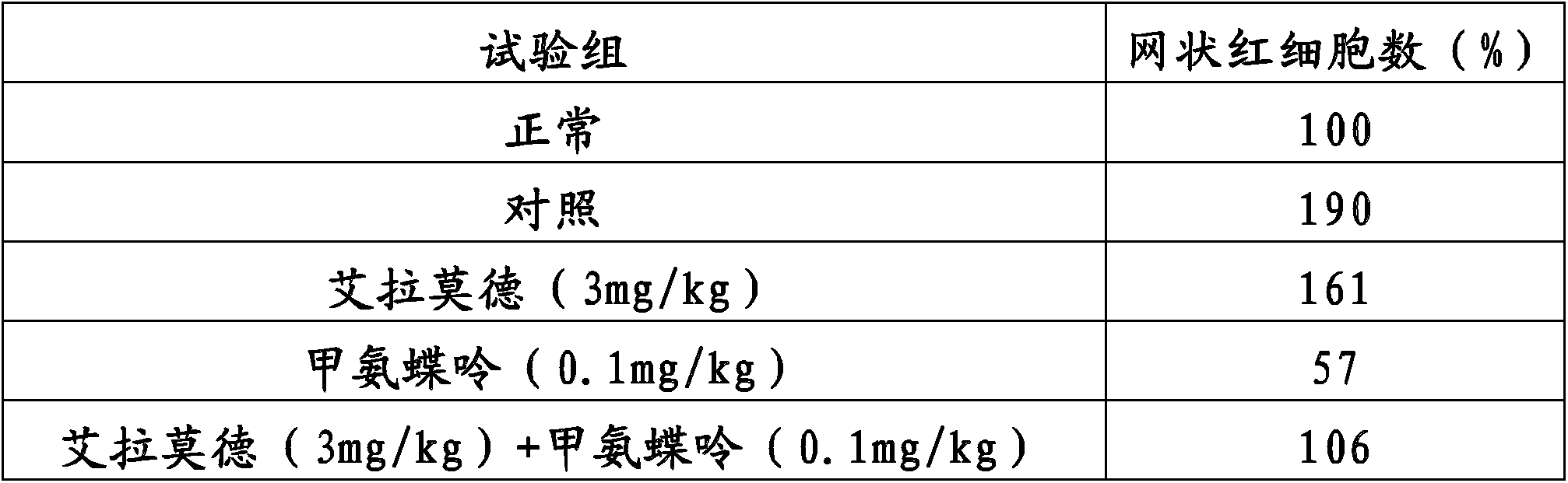
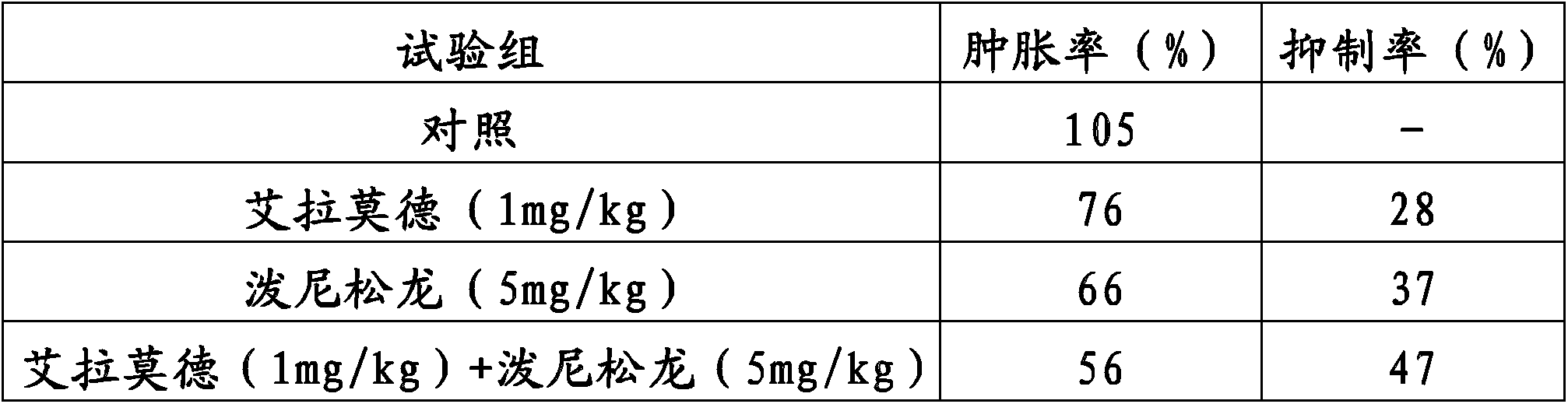
Method for improving therapy for autoimmune diseases such as rheumatoid arthritis
InactiveCN103826624AOrganic active ingredientsOrganic chemistryAutoimmune responsesAutoimmune thyroid disease
In the present invention, a method using a combination of iguratimod or a salt thereof and one or more immunosuppressants is useful as a method for the treatment of autoimmune diseases, and with this method adverse effects are lessened. A pharmaceutical composition containing this combination is useful for the treatment of autoimmune diseases. This method and pharmaceutical composition are useful for the treatment of more severe autoimmune diseases.
Owner:TOYAMA CHEM CO LTD
Preparation method of Iguratimod formylation intermediates
ActiveCN108727232AQuick responseImprove conversion rateSulfonic acid amide preparationSide reactionImpurity
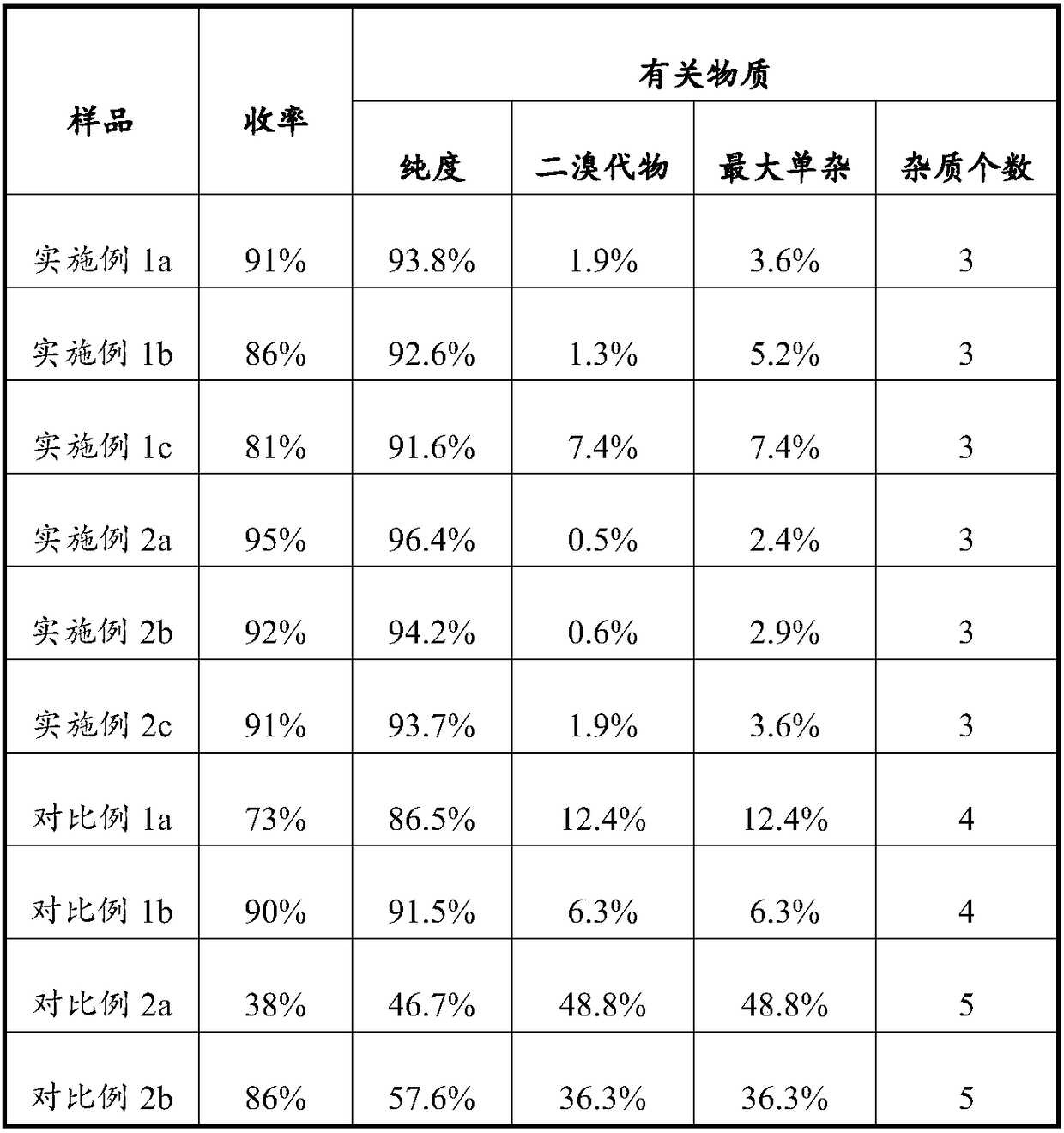
The invention relates to the technical field of synthesis of chemical drugs, in particular to a preparation method of Iguratimod formylation intermediates. The preparation method comprises the following steps: mixed acid anhydride is prepared from formic acid, sodium formate and pivaloyl chloride, a compound I is added for carbamylation, and the Iguratimod formylation intermediates are obtained. Mixed acid anhydride is prepared from formic acid and pivaloyl chloride, the reaction with pivaloyl chloride is a homogeneous reaction, reaction speed is high, and conversion rate is high; the relatively acidic environment of the system is kept by formic acid, side reactions in which piperazine compounds are generated from alpha-aminoketone compounds in raw materials through own condensation reaction under alkaline conditions are avoided, yield is increased, impurities are reduced, and purity is improved. The Iguratimod formylation intermediates prepared with the method are high in purity and can be used for preparing Iguratimod.
Owner:康美(北京)药物研究院有限公司 +2
Iguratimod osmotic pump controlled slow-release preparation
InactiveCN101564382AStable and controllable qualityStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismBlood concentrationCellulose acetate
The invention relates to a slow-release control preparation containing iguratimod and a preparation method thereof. The slow-release control preparation is an osmotic pump type double-layer tablet using osmotic pressure of an osmotic pump as main release power. The preparation consists of a double-layer tablet core, a semi-permeable membrane and a release pore, and contains high molecular materials with slow-release control function such as polyoxyethylene, cellulose acetate and the like. Compared with the common tablet, the preparation has more durable release performance and more stable blood concentration, and only needs to be taken once every day. The preparation provides better medicament selection for the treatment of rheumatoid arthritis, and reduces the risk of adverse reaction possibly caused by the fluctuation of the iguratimod blood concentration. The preparation is orally-taken slow-release control preparation, and has stable and controllable quality; and the prescription and preparation processes are reliable and feasible, and are suitable for industrialized production.
Owner:JIANGSU SIMCERE PHARMACEUTICAL R & D CO LTD +1
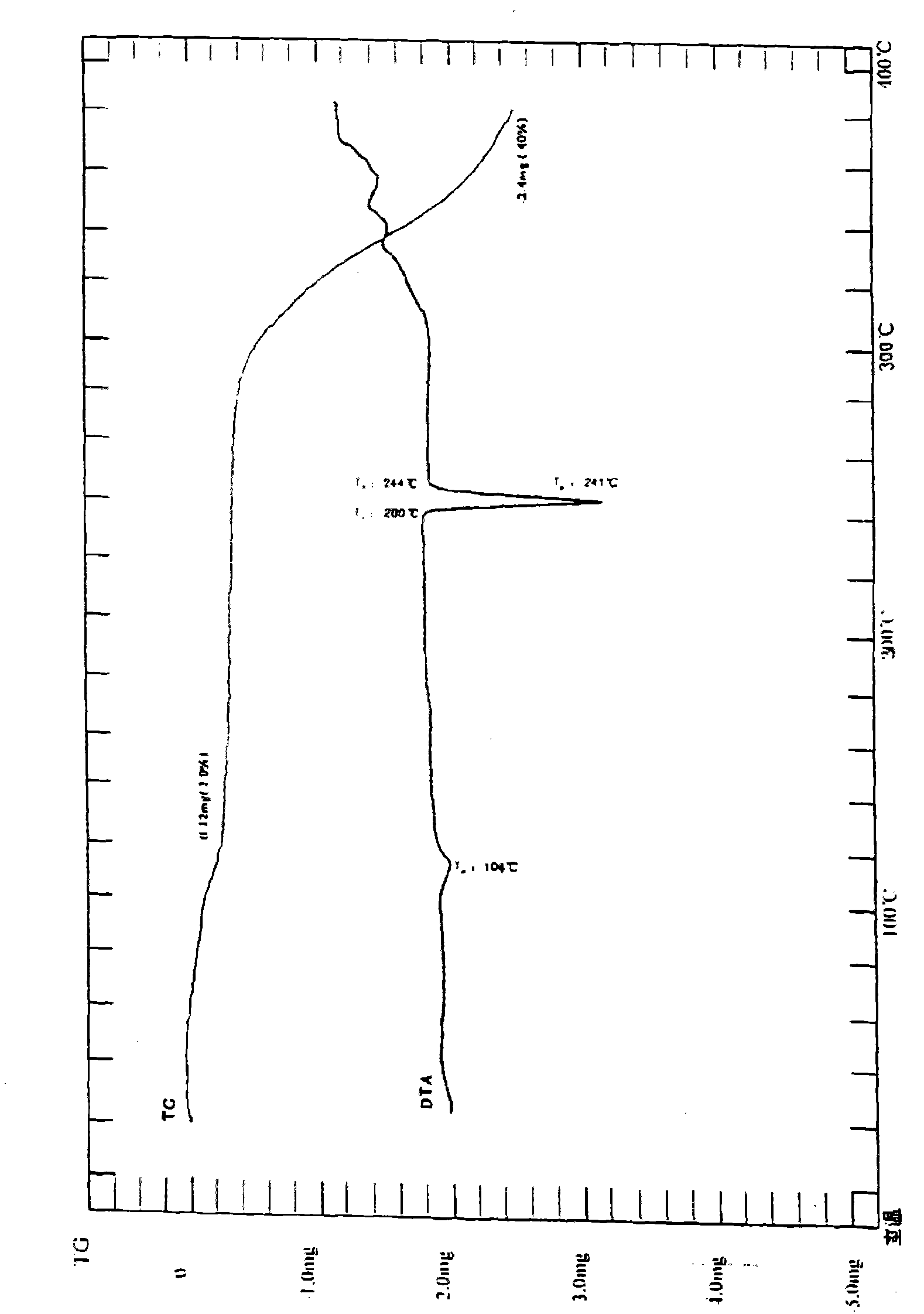
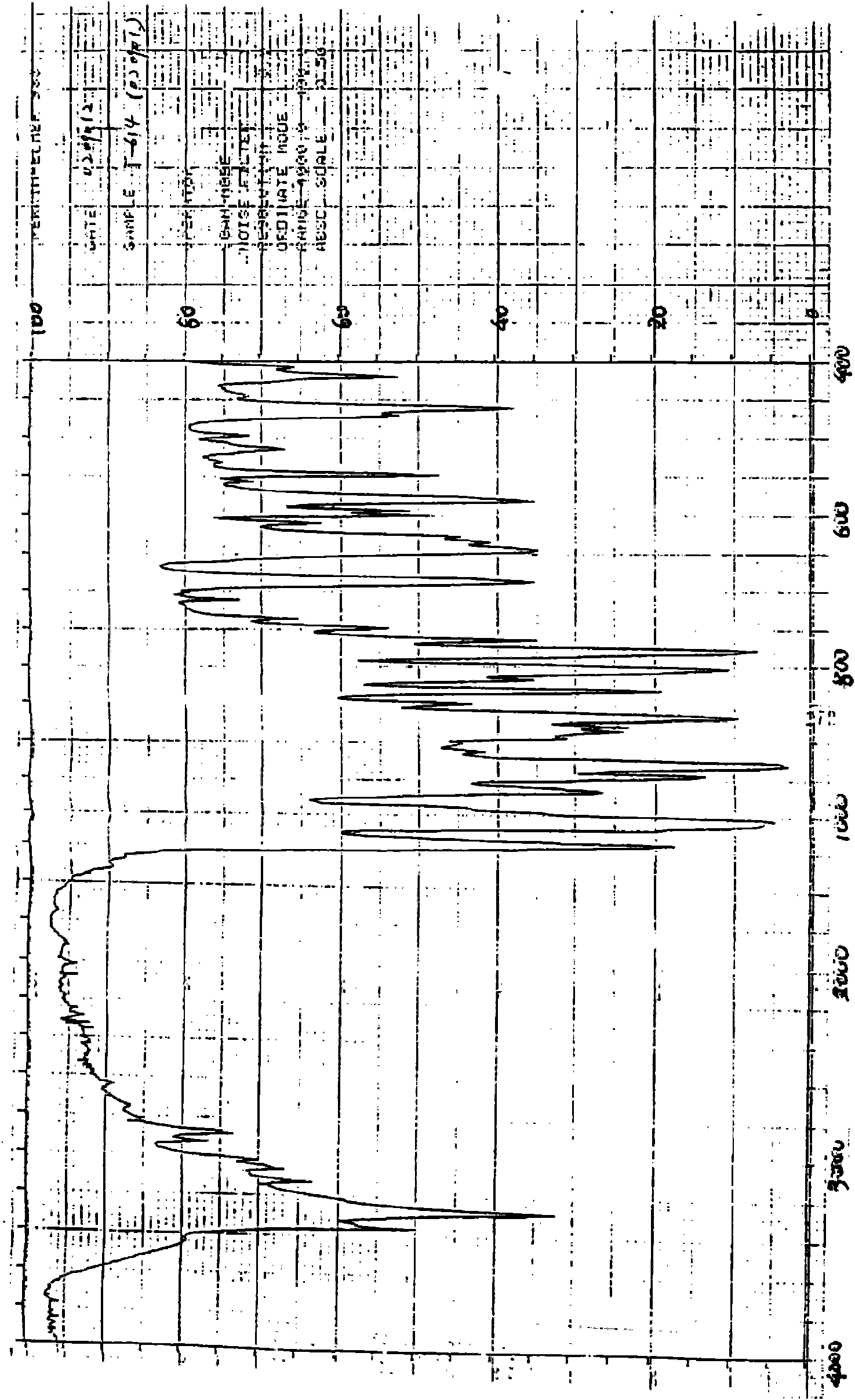
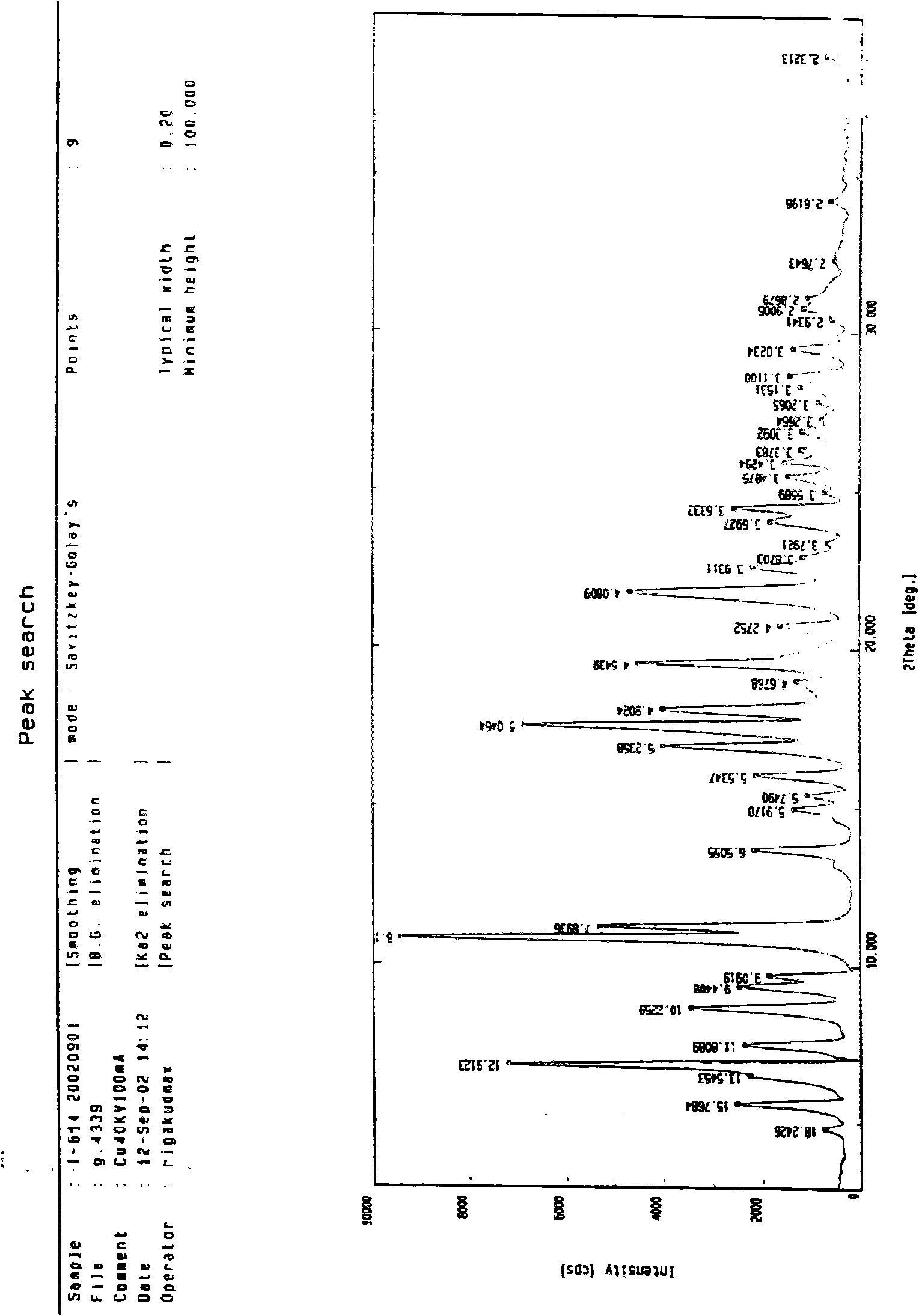
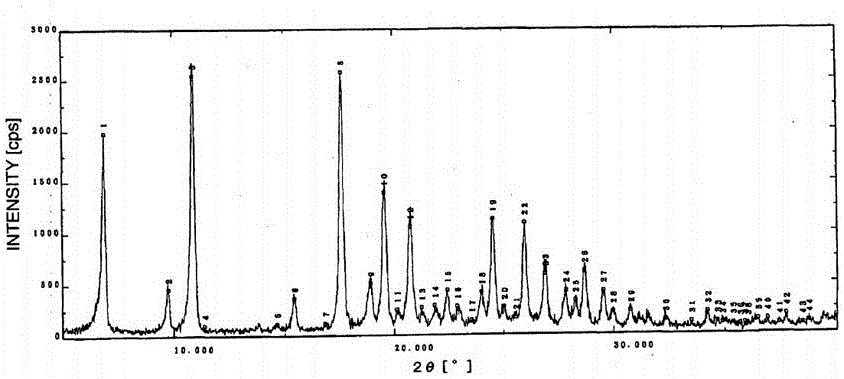
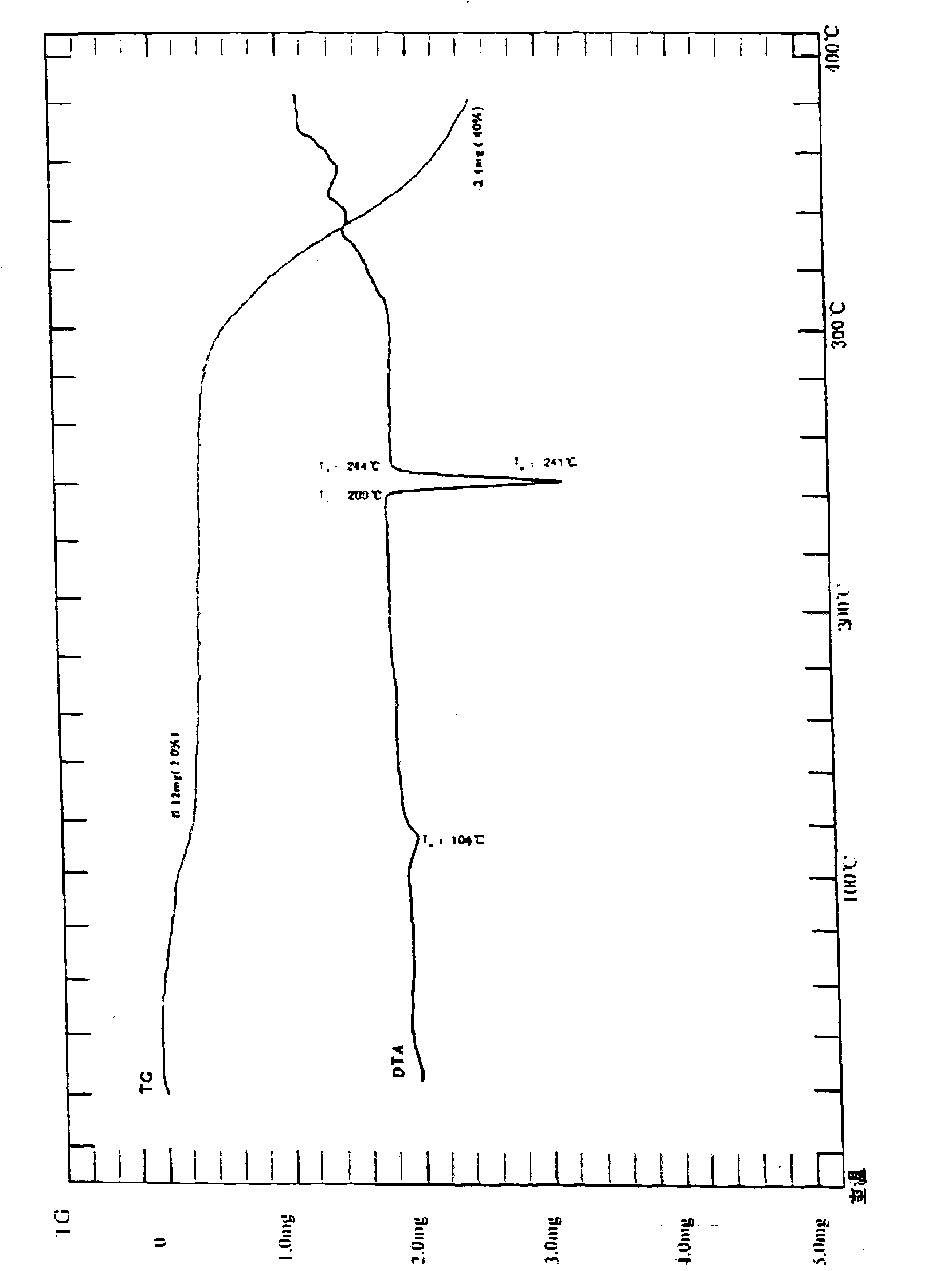
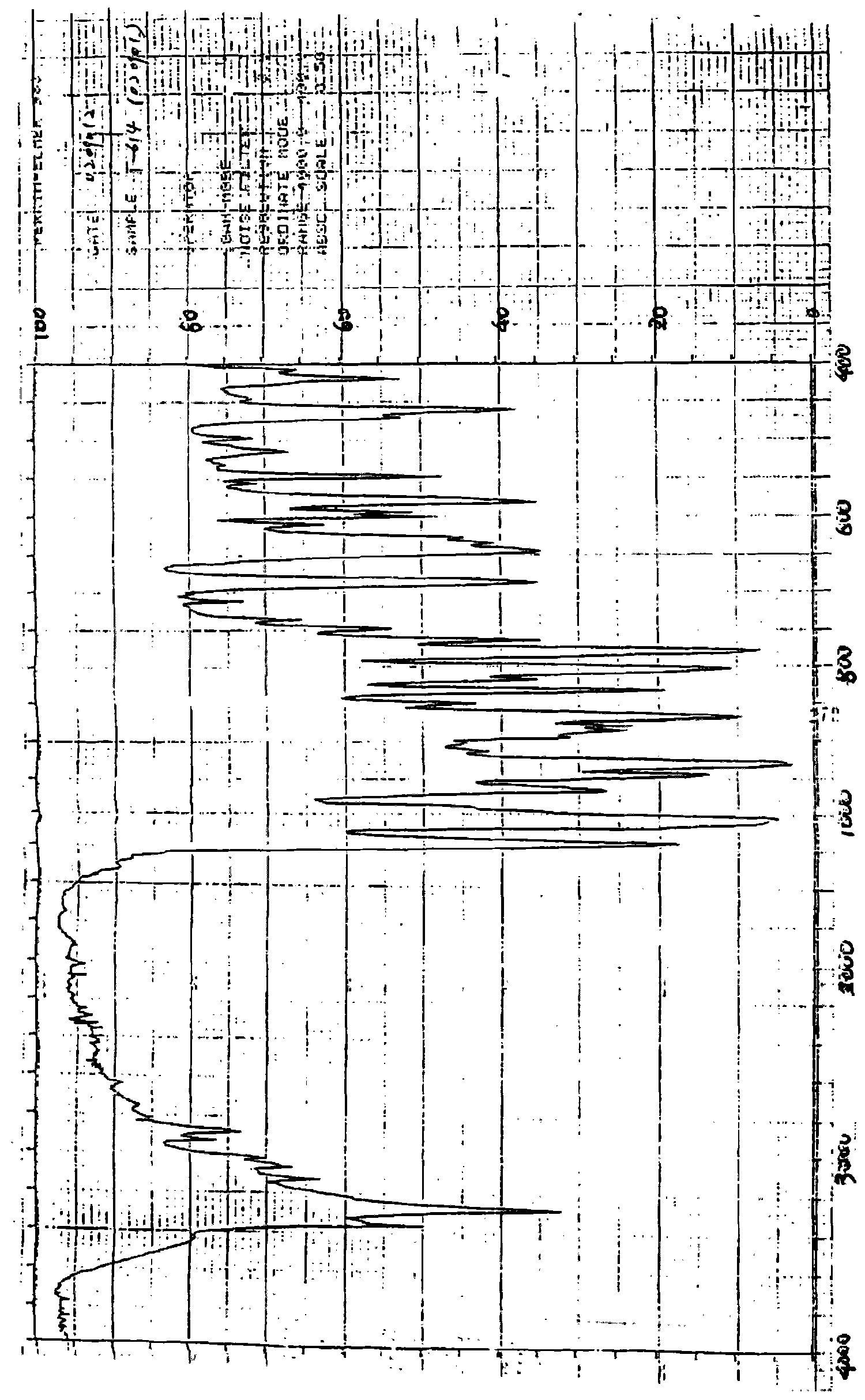
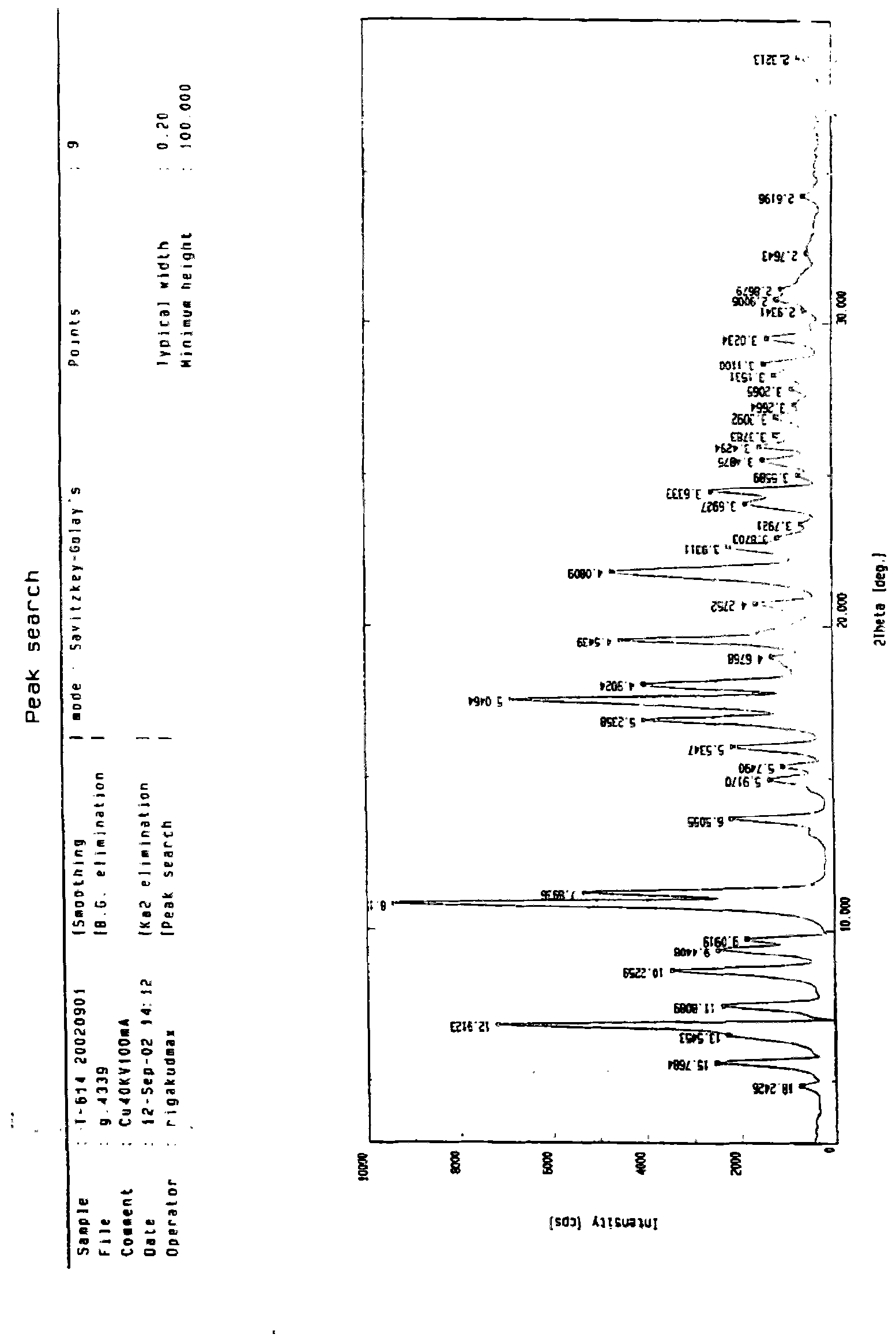
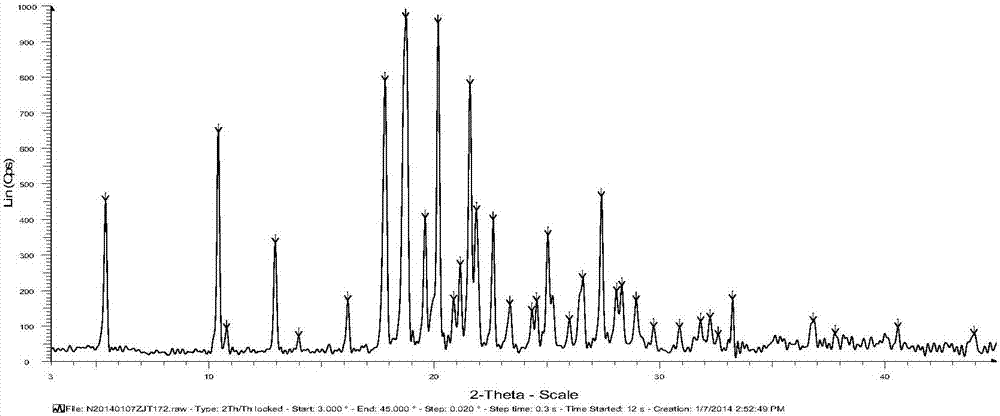
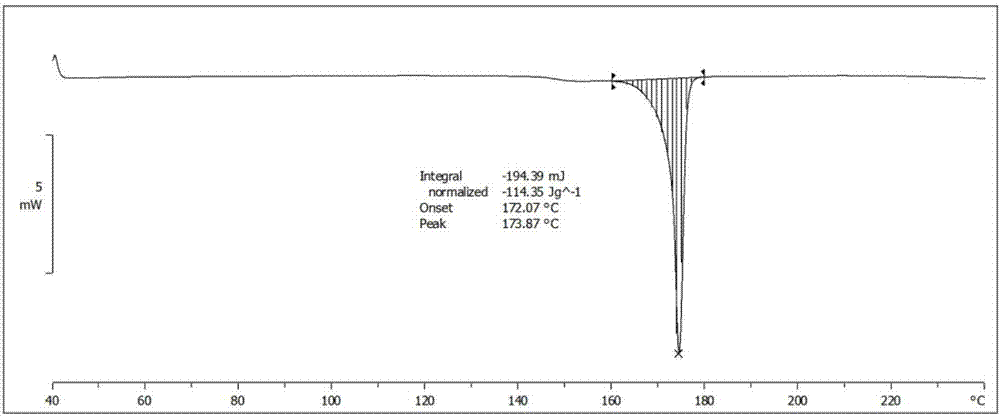
Iguratimod crystal habit and composition thereof
ActiveCN101885717AHigh melting pointQuality improvementOrganic active ingredientsOrganic chemistryBenzopyranMedicine
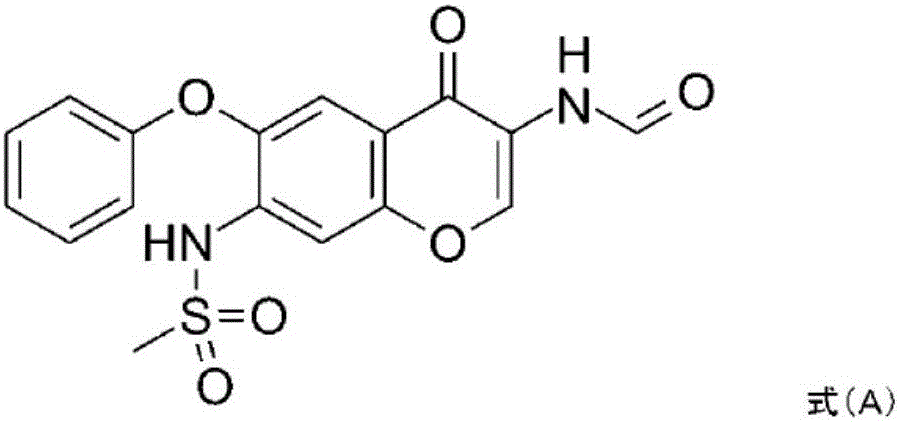
The invention relates to a crystal habit (iguratimod) of N-[3- (formamido)-4-oxygen-6-phenoxy-4H-1- benzopyran-7-base]-methylsulfonamid. In addition, the invention also relates to application of iguratimod crystal habit to medicine for treating rheumatoid arthritis, a medicine composition containing the iguratimod crystal habit and a preparation method of the iguratimod crystal habit.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +2
Preparation method of alpha crystal form of Iguratimod
The invention discloses a preparation method of an alpha crystal form of Iguratimod. A preparation process is optimized with the method, an ethanol / DMF mixed solvent with better solubility is used, the use quantity of the solvent is reduced, production time is shortened, the cost is reduced, and the method can be applied to industrial production.
Owner:JIANGSU QINGJIANG PHARMA
Medicinal composition capable of preventing or treating inflammatory diseases
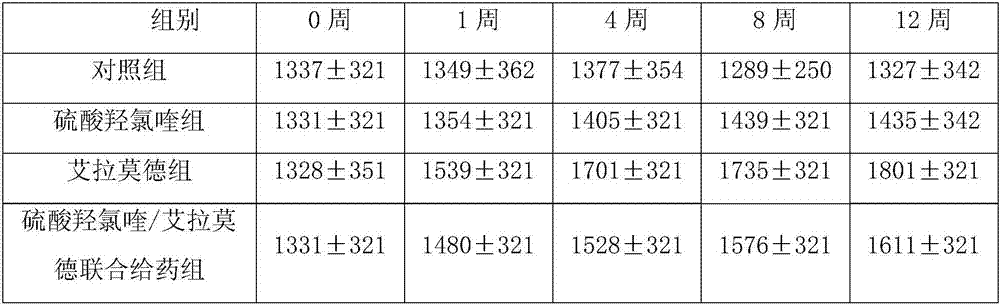
The invention discloses a medicinal composition which comprises hydroxychloroquine and iguratimod, and further relates to applications of hydroxychloroquine and salts of hydroxychloroquine in preparing medicines capable of alleviating the toxic and side effects of iguratimod. The compound composition has the obvious effects of synergists and the obvious effect of reducing toxic and side effects.
Owner:HAINAN SIMCERE PHARMA CO LTD +1
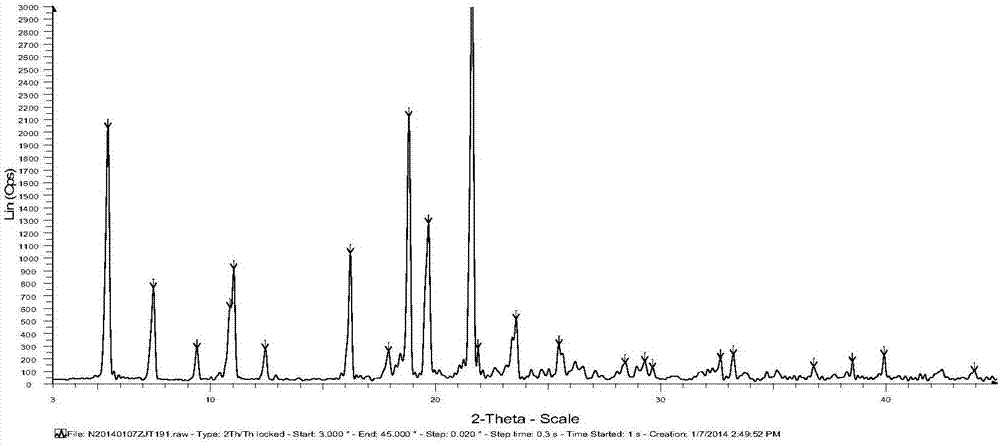
Iguratimod crystalline form and composite thereof
ActiveCN101891726AHigh melting pointQuality improvementOrganic active ingredientsOrganic chemistryMedicineCrystallization
The invention relates to a crystalline form (Iguratimod) of N-[3-(formylamino)-4-oxo-6-phenoxy-4H-1-benzopyran-7-yl]-methyl sulfonamide. In addition, the invention further relates to application of the Iguratimod crystalline form in the medicine of treating rheumatoid arthritis, a pharmaceutical composition containing Iguratimod crystalline form and a method for preparing crystalline Iguratimod.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +2
Method for improving therapy for autoimmune diseases such as rheumatoid arthritis
ActiveUS20150080356A1Reduce adverse reactionsGood effectBiocideOrganic chemistryAutoimmune thyroid diseaseAutoimmune responses
In the present invention, a method using a combination of iguratimod or a salt thereof and one or more immunosuppressants is useful as a method for the treatment of autoimmune diseases, and with this method adverse effects are lessened. A pharmaceutical composition containing this combination is useful for the treatment of autoimmune diseases. This method and pharmaceutical composition are useful for the treatment of more severe autoimmune diseases.
Owner:TOYAMA CHEM CO LTD
The use of actarit in the prophylaxis or treatment of renal fibrosis or kidney disease
PendingUS20210260000A1Reduction in renal fibrosisPeptide/protein ingredientsUrinary disorderEmoxypineRepirinast
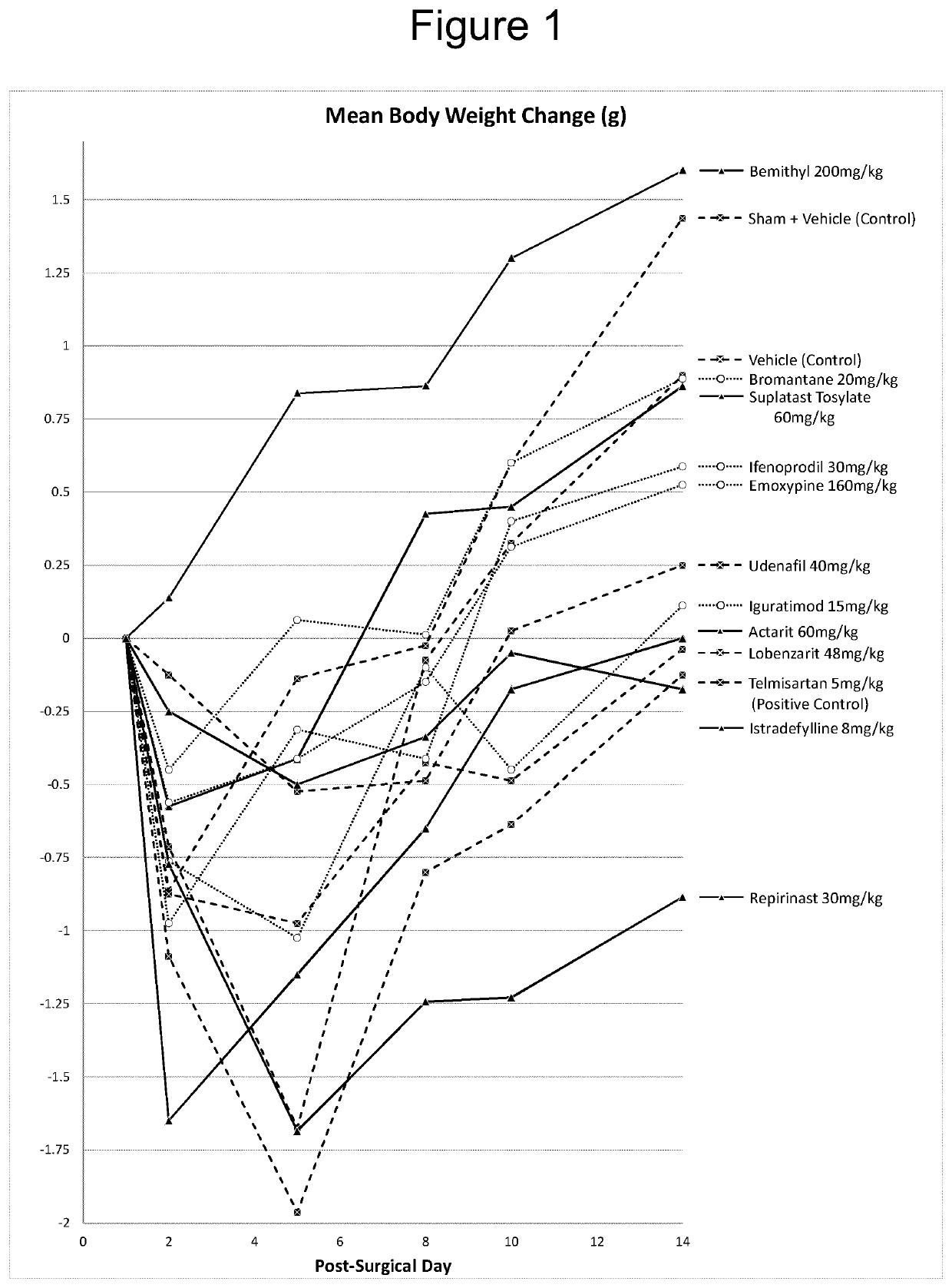
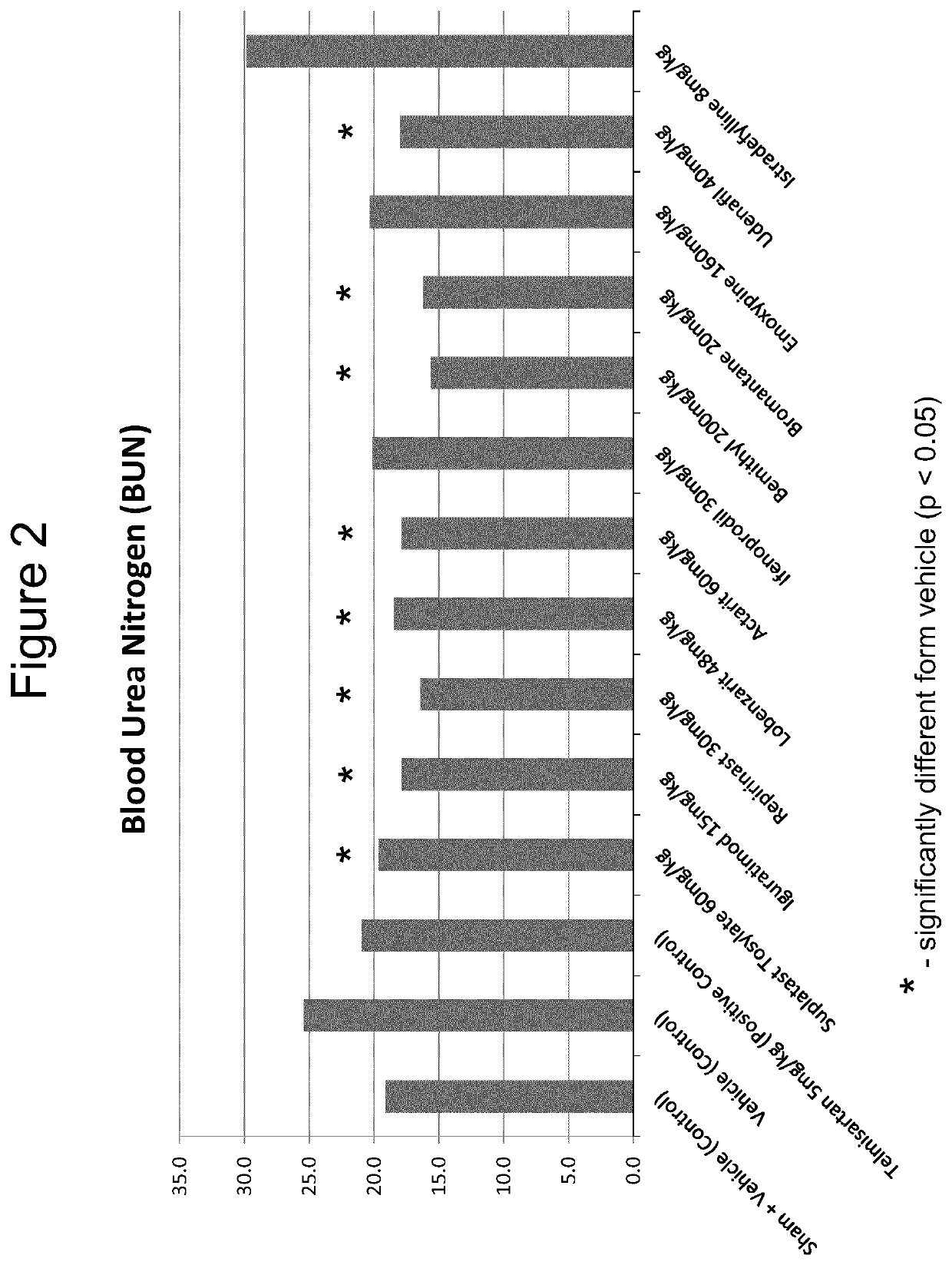
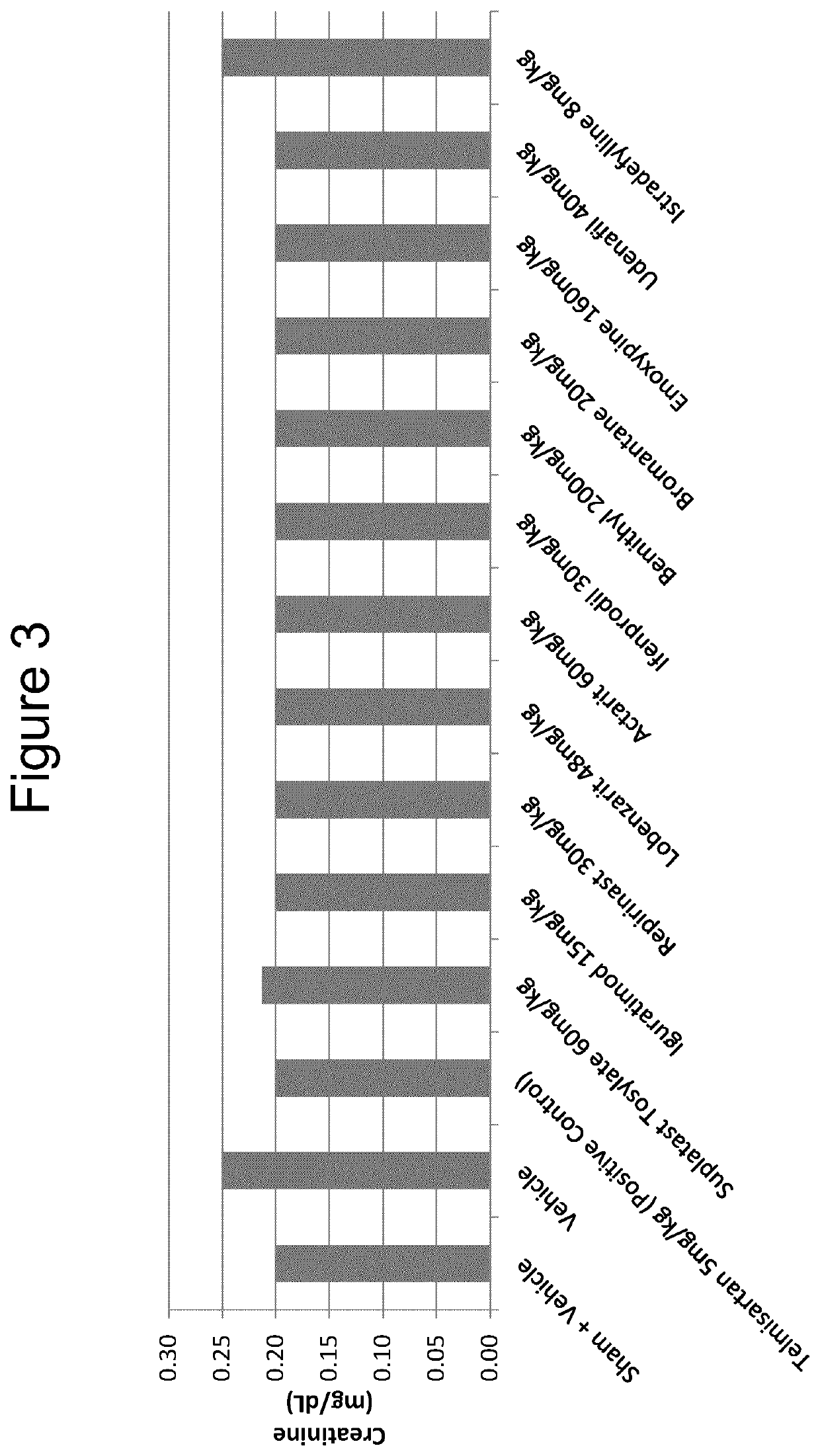
Iguratimod, Repirinast, Lobenzarit, Actarit, Ifenprodil, Bemithyl, Bromantane, Emoxypine, Udenafil, and / or Istradefylline are used for the treatment or prophylaxis of renal fibrosis, kidney disease, or chronic kidney disease in a subject.
Owner:ALGERNON PHARMA INC
Preparation method of Iguratimod intermediate
PendingCN114539104AEasy to getPromote environmental protectionOrganic compound preparationSulfonic acid amide preparationP-nitroanisolePtru catalyst
The invention discloses a preparation method of an Iguratimod intermediate, which comprises the following steps: by taking p-nitroanisole as a raw material, carrying out substitution nucleophilic substitution (VNS) on p-nitroanisole and methoxyamine hydrochloride in the presence of a copper salt catalyst and an acid-binding agent to generate 5-methoxy-2-nitroaniline (compound II); the synthesis method comprises the following steps: carrying out a nucleophilic substitution reaction on 5-methoxy-2-nitroaniline (compound II) and methanesulfonyl chloride to generate a compound III, etherifying the compound III and phenol under the catalysis of a copper salt to generate N-(5-methoxy-2-phenoxy phenyl) methane sulfonamide (compound IV), and reagents used in the synthesis process are non-highly toxic products and are easy to obtain; no iron powder is used in the reaction process, so that iron mud which is harmful to the environment is not generated, and the environmental protection property is high; the reaction operation difficulty is small, the safety is high, and a foundation is laid for industrial preparation of Iguratimod drugs.
Owner:常州佳德医药科技有限公司
Sodium salt compound of Iguratimod, preparation method thereof and pharmaceutical use thereof
The invention relates to a sodium salt compound of Iguratimod and a hydrate or solvate thereof. The slat of the Iguratimod is far advantageous over the Iguratimod in water solubility, powder flowability and power stability. The compounds can be used in the preparation of medicaments for treating diseases such as rheumatoid arthritis, chronic infectious arthritis, osteoarthritis and ankylosing spondylitis and relieving pains caused by muscle or soft tissue damages and arthralgia. The invention also provides a preparation method of the sodium salt compound and the hydrate or solvate thereof, the use (medicinal compositions and medicinal composition preparation and use) of the compounds in medicaments, and the like.
Owner:杨喜鸿
Preparation method of iguratimod intermediate
ActiveCN112209859AReduce pollutionIn line with the concept of green technologySulfonic acid amide preparationAcyl groupHigh activity
The invention discloses a preparation method of an iguratimod intermediate. The preparation method comprises the following steps: by using formic acid as a starting raw material, subjecting formic acid to reacting with N,N-carbonyldiimidazole (CDI) via an active ester method to obtain formylimidazole with higher activity, and subjecting the formylimidazole to reacting with 2-amino-1-(2-methoxy-4-methanesulfonamido-5-phenoxyphenyl)ethanone hydrochloride to obtain an important intermediate compound of iguratimod. The method has the advantages of being safe, environmentally friendly, easy to operate, high in yield, good in product purity, suitable for industrial production and the like, and is suitable for preparing the iguratimod intermediate.
Owner:江苏润安制药有限公司
Prevention or treatment agent for cerebral amyloid-beta storage diseases
InactiveCN106102736AReduce accumulationStop progressOrganic active ingredientsNervous disorderAmyloid betaIguratimod
Owner:FUJIFILM RI PHARMA
Method for improving therapy for autoimmune diseases such as rheumatoid arthritis
ActiveUS9421186B2Reduce adverse reactionsGood effectBiocideOrganic chemistryAutoimmune responsesAutoimmune disease
In the present invention, a method using a combination of iguratimod or a salt thereof and one or more immunosuppressants is useful as a method for the treatment of autoimmune diseases, and with this method adverse effects are lessened. A pharmaceutical composition containing this combination is useful for the treatment of autoimmune diseases. This method and pharmaceutical composition are useful for the treatment of more severe autoimmune diseases.
Owner:TOYAMA CHEM CO LTD
A kind of preparation method of iguratimod formylation intermediate
ActiveCN108727232BQuick responseEasy to operateSulfonic acid amide preparationPharmaceutical SubstancesSide reaction
The invention relates to the technical field of synthesis of chemical drugs, in particular to a preparation method of Iguratimod formylation intermediates. The preparation method comprises the following steps: mixed acid anhydride is prepared from formic acid, sodium formate and pivaloyl chloride, a compound I is added for carbamylation, and the Iguratimod formylation intermediates are obtained. Mixed acid anhydride is prepared from formic acid and pivaloyl chloride, the reaction with pivaloyl chloride is a homogeneous reaction, reaction speed is high, and conversion rate is high; the relatively acidic environment of the system is kept by formic acid, side reactions in which piperazine compounds are generated from alpha-aminoketone compounds in raw materials through own condensation reaction under alkaline conditions are avoided, yield is increased, impurities are reduced, and purity is improved. The Iguratimod formylation intermediates prepared with the method are high in purity and can be used for preparing Iguratimod.
Owner:康美(北京)药物研究院有限公司 +2
Prevention or treatment agent for cerebral amyloid beta storage diseases
ActiveUS9968585B2Inhibit progressImprove the quality of lifeOrganic active ingredientsNervous disorderAmyloid betaAlzheimer type dementia
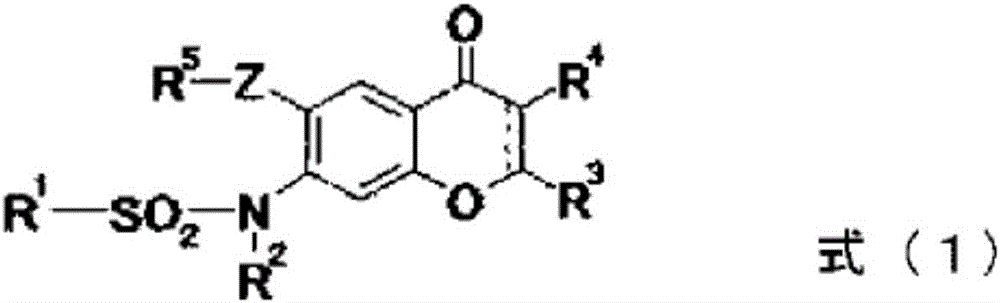
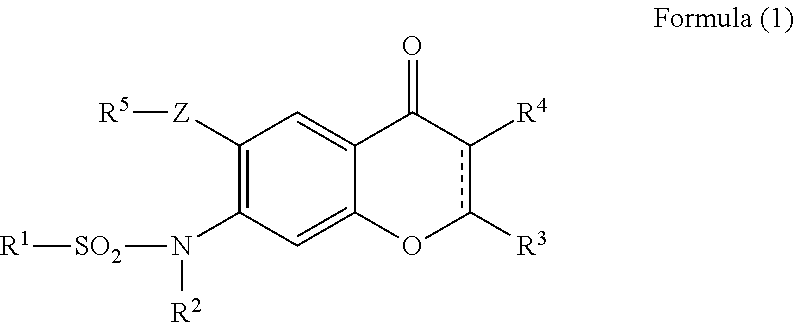
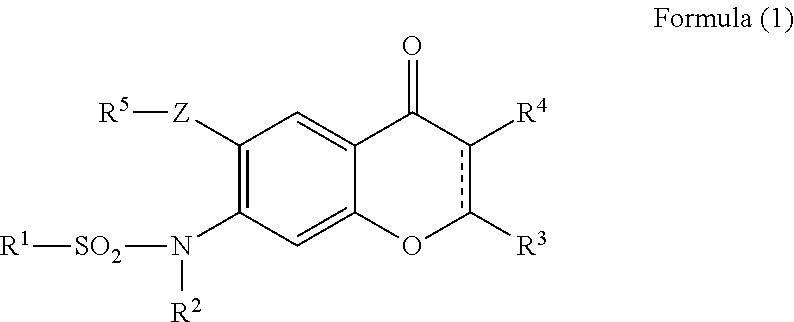
Provided is a prevention or treatment agent for cerebral amyloid beta storage diseases, that contains a substance capable of suppressing the progression, alleviating the symptoms, and improving cerebral amyloid beta storage diseases. This prevention or treatment agent for cerebral amyloid beta storage diseases has as an effective component thereof a compound (e.g., Iguratimod) indicated by formula (1) or a salt thereof and, as a result, is capable of preventing or treating cerebral amyloid beta storage diseases such as Alzheimer-type dementia or cerebral amyloid angiopathy.
Owner:TOYAMA CHEM CO LTD
Preparation method of iguratimod intermediate IV
InactiveCN107162942AReduce harmReduce pollutionSulfonic acid amide preparationChemical industryNitrobenzene
Owner:常州佳德医药科技有限公司
Method for preparing high-yield and high-purity Iguratimod intermediate
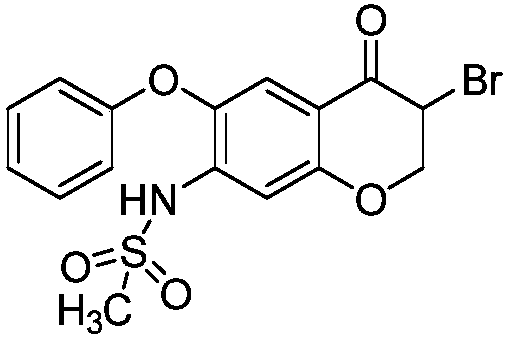
The invention discloses a method for preparing an Iguratimod intermediate, i.e., 3-bromo-7-methsulfonamido-6-phenoxy-4H-1-benzo-2,3-dihydropyran-4-one. The method comprises the following steps: (1) dissolving cupric bromide in ethyl acetate and C1-C4 lower alkanol, and carrying out stirring for at least 30 minutes; (2) adding a dichloromethane solution of 7-sulfonamido-6-phenoxy-4H-1-benzo-2,3-dihydropyran-4-one into the solution obtained in the step (1), heating the temperature of the mixture to 20 DEG C to 50 DEG C with stirring, and performing a reaction for 2 to 6 hours; (3) carrying out filtering, washing filter liquor with an aqueous solution of sodium thiosulfate, and concentrating an organic phase, so as to obtain a crude product of the intermediate; and (4) carrying out refining with dichloromethane and petroleum ether, thereby obtaining a refined intermediate. The method is convenient in operation, high in conversion ratio and high in product purity, and the purity can reach96% or more.
Owner:YANGTZE RIVER PHARM GRP CO LTD
The use of actarit in the prophylaxis or treatment of renal fibrosis or kidney disease
Owner:ALGERNON PHARMA INC
Iguratimod crystal habit and composition thereof
The invention relates to a crystal habit (iguratimod) of N-[3- (formamido)-4-oxygen-6-phenoxy-4H-1- benzopyran-7-base]-methylsulfonamid. In addition, the invention also relates to application of iguratimod crystal habit to medicine for treating rheumatoid arthritis, a medicine composition containing the iguratimod crystal habit and a preparation method of the iguratimod crystal habit.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +2
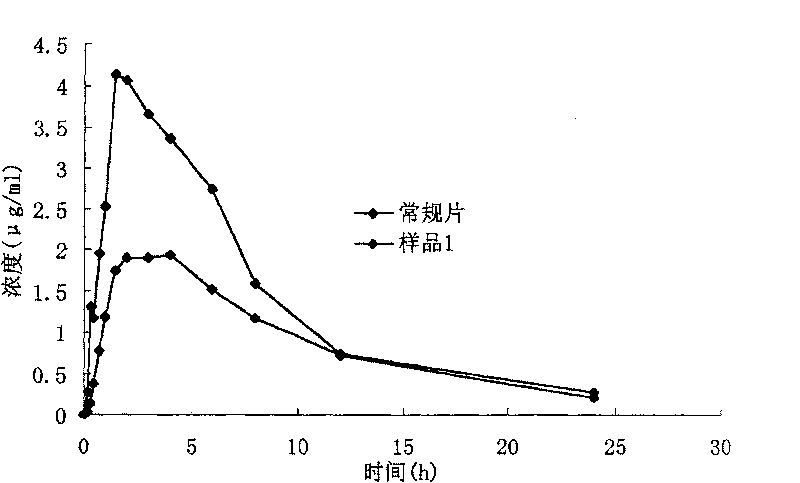
Iguratimod oral double-layer sustained-release preparation
InactiveCN101095671BMake up for the shortcomings of short action time and slow onsetEffective plasma concentrationOrganic active ingredientsAntipyreticSide effectEffective action
The invention relates to oral double iguratimod controlled release formulation, which comprises fast release layer and slow release layer that are composed of 8-30% micronizing iguratimod crystal powder and medical findings, and the granule size of iguratimod crystal powder is 1-10 um. The effective component in fast release layer is released in short time and reaches to effective blood chemical concentration for effective action; the iguratimod in sloe release layer is released gradually and maintains effective blood medical concentration for continuous effective action. The invention overcomes shortcomings of short effective action time and a little high toxic effect.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
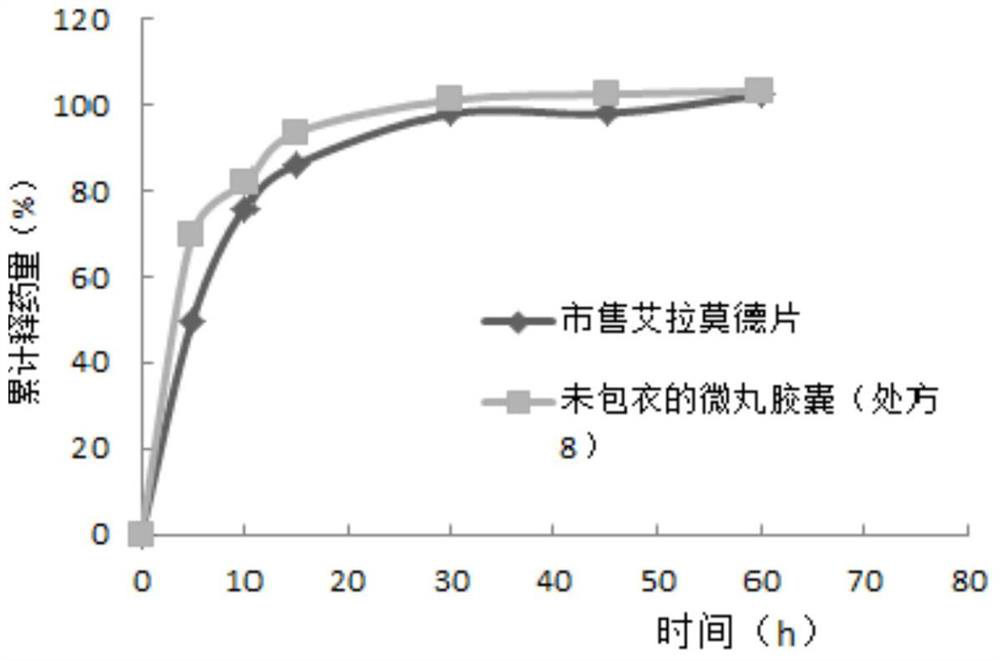
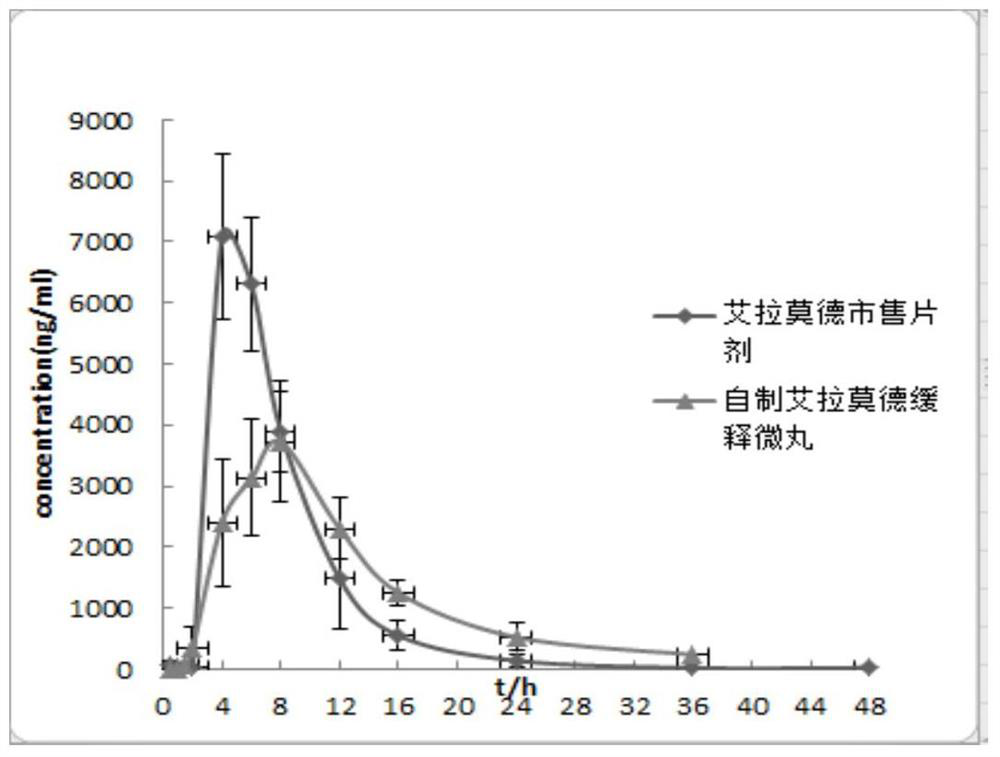
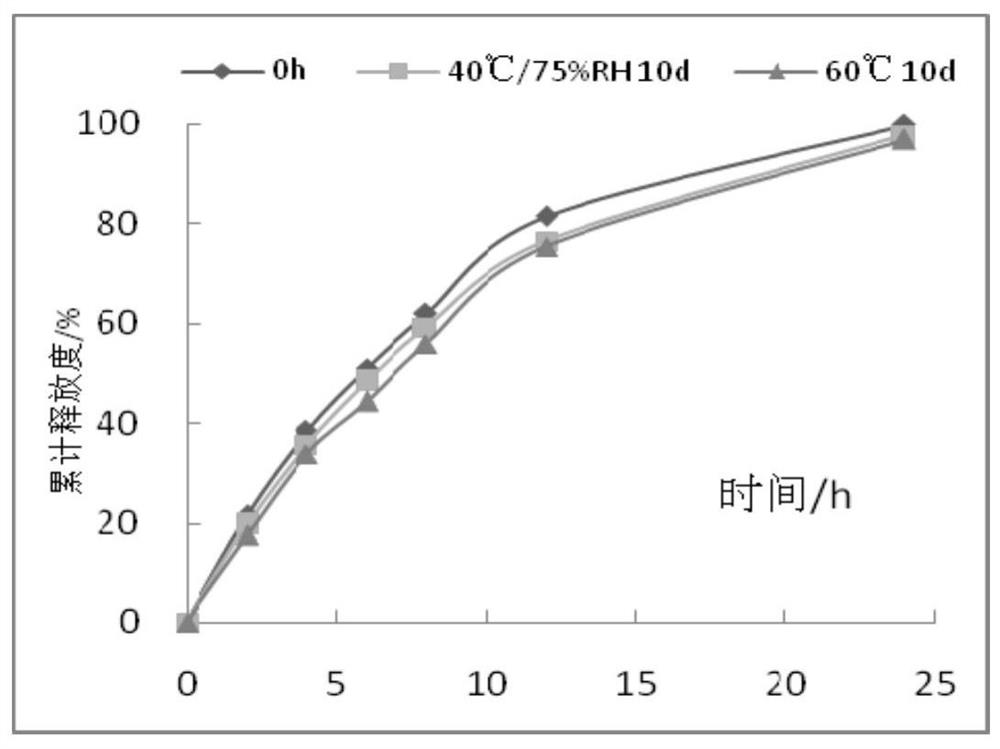
Iguratimod sustained-release capsules and preparation method thereof
The invention relates to an iguratimod sustained-release capsule and a preparation method thereof. The iguratimod sustained-release capsules of the present invention are made of iguratimod sustained-release pellets and then filled with capsules. The iguratimod-containing main drug layer and the isolation layer outside the pellet core are composed of a slow-release coating layer. The technology of the iguratimod sustained-release capsules of the present invention is simple and easy to implement, has good reproducibility, has obvious sustained-release properties, can maintain a relatively stable blood drug concentration and a longer action time, and greatly improves the safety of the drug , compliance, avoiding the disadvantages of frequent administration, shortening the treatment time and the number of consultations, and reducing the cost of treatment.
Owner:JIANGSU SIMCERE PHARMA +1
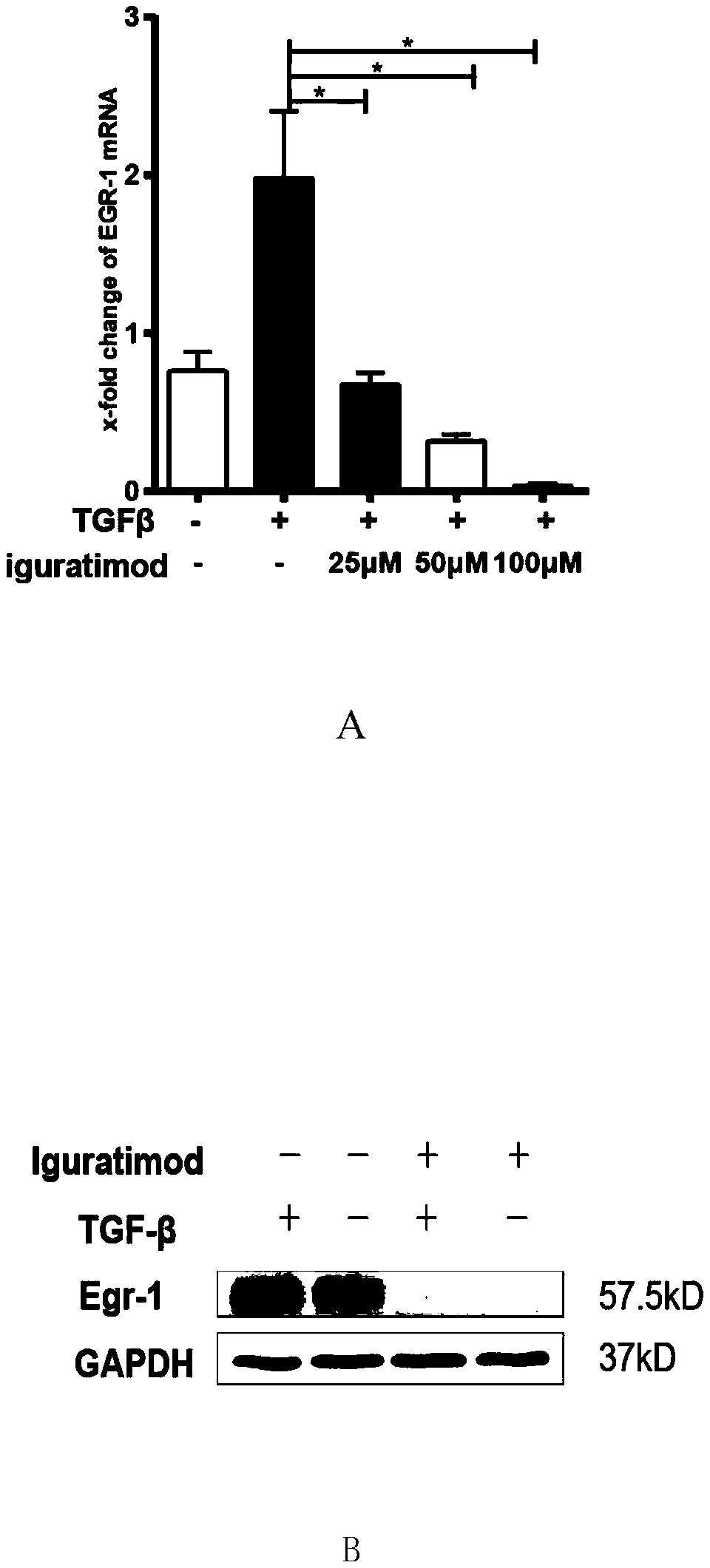
Application of iguratimod in preparation of medicine for treating systemic sclerosis
InactiveCN110638808APositive and obvious technical effectOrganic active ingredientsImmunological disordersFibrosisPharmaceutical drug
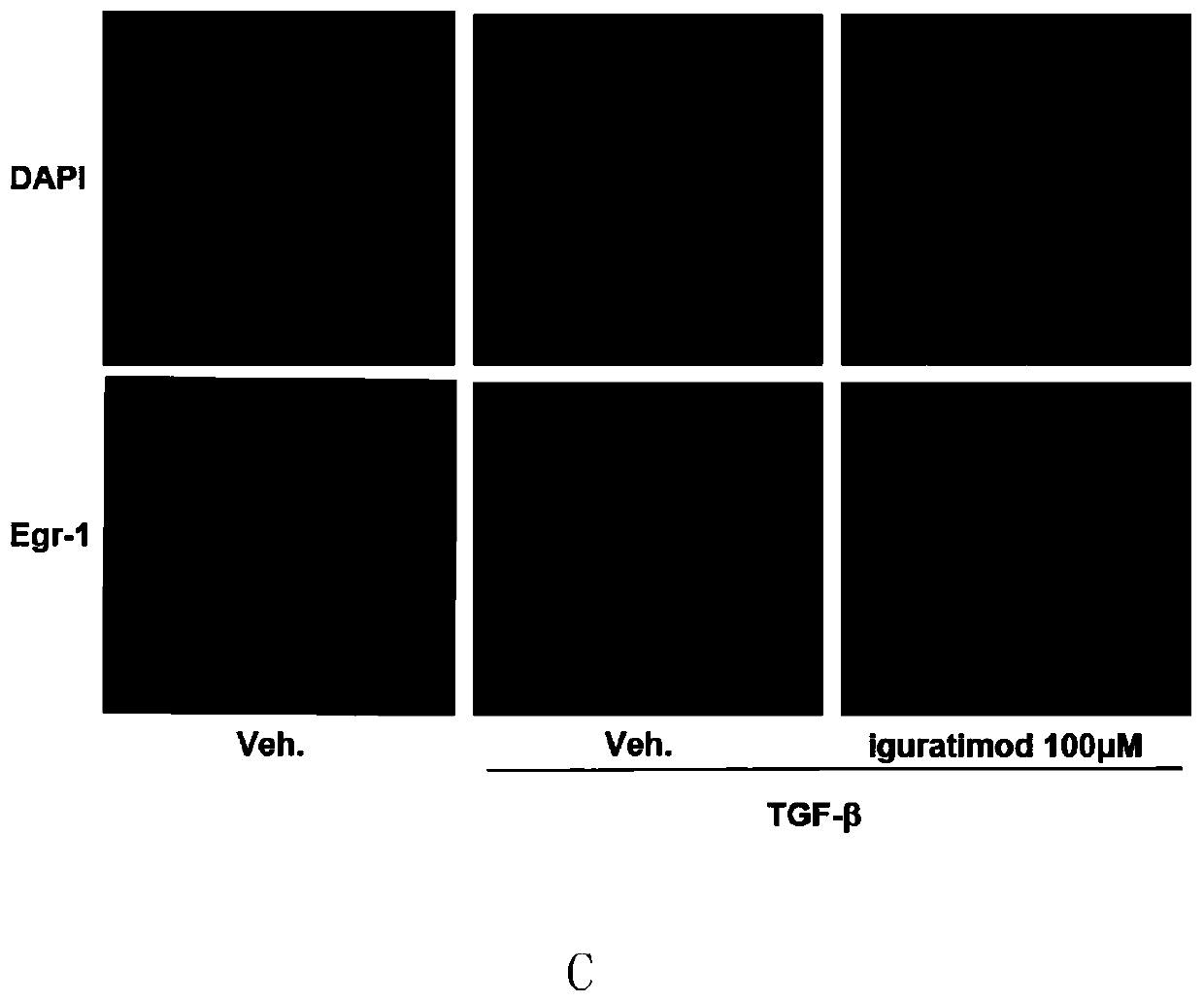
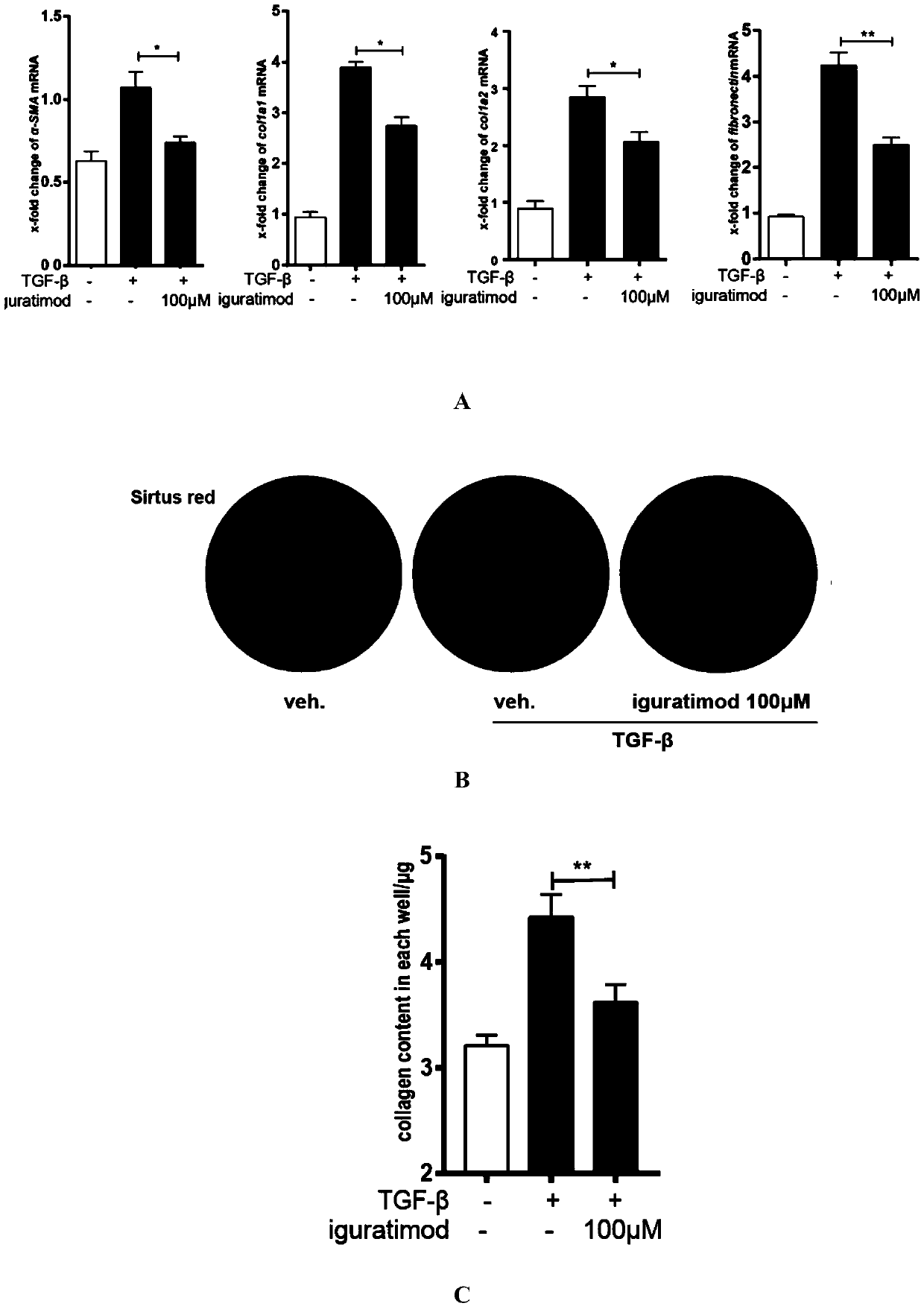
The invention provides an application of iguratimod in preparation of a medicine for treating systemic sclerosis. Inventors find that the iguratimod has application potential that an external dosage form for external use of the iguratimod can be prepared, and hardened skin can be softened by topical application. Experiments prove that a local or systemic application of the iguratimod can significantly inhibit important transcription factor EGR1 involved in fibrosis to decrease expression of pro-fibrogenic cytokine TGF-beta or decrease signals in downstream, thereby inhibiting the progression of the systemic sclerosis. The topical application of an iguratimod dimethyl sulfoxide solution can also effectively reduce and cure experimental scleroderma skin fibrosis.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Production method of iguratimod intermediate
InactiveCN112375018AReduce generationSimple processOrganic compound preparationSulfonic acid amide preparationAcetophenoneSodium salt
The invention discloses a production method of an iguratimod intermediate, which comprises the following steps: S1, by using alpha-amino-2-methoxy-4-methanesulfonylamino-5-phenoxy acetophenone hydrochloride as a starting raw material, selecting a 100L reaction kettle, and adding 27kg of acetone and 1kg of sodium carbonate into the reaction kettle, wherein pivaloyl chloride is taken as an acylatingagent. The preparation method is simple in process and low in production cost, that is, sodium carbonate is added into the reaction raw material to generate impurities in the reaction process of pivaloyl chloride and sodium formate to generate sodium salt, so that the impurities cannot continuously react with alpha-amino-2-methoxy-4-methanesulfonylamino-5-phenoxy acetophenone hydrochloride, the generation of reaction impurities is reduced, the purity of the product is improved, the product is not refined to obtain a target product, and the yield is 85-95%.
Owner:WUDI REACTION PHARMA & CHEM
Superfine Iguratimod powder and quick released oral preparation
InactiveCN1931159BAccelerate time to peak blood concentrationImprove in vitro dissolutionPowder deliveryOrganic active ingredientsActive componentDiluent
The present invention relates to the micronizing technology for insoluble polycrystal medicine Iguratimod, and the micronized Iguratimod has new crystallization and powder size of 1-10 microns. The orally taken quick release preparation with micronized Iguratimod as active component, high efficiency disintegrant, surfactant, diluent, etc has obviously improved in vitro leaching rate and in vivo absorption rate, and obviously raised bioavailability.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Iguratimod as an mif inhibitor
InactiveUS20190262307A1Reduce doseIncrease successOrganic active ingredientsDigestive systemDiseaseDepressant
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Crystalline morphology of Iguratimod intermediate VI
InactiveCN106957249AHigh melting pointQuality improvementOrganic chemistry methodsSulfonic acid amide preparationMedicinal chemistryCrystallization
The invention belongs to the technical field of pharmaceutical chemical engineering, and relates to a crystalline morphology of an Iguratimod intermediate VI (alpha-formamido-2-hydroxy-4-methanesulfonamide-5-phenoxyacetophenone). In addition, the invention further relates to a preparation method for two types of crystalline morphologies of Iguratimod intermediates VI. The two different types of crystalline morphologies of Iguratimod intermediates VI provided by the invention have good melting point and quality, the yield by weight is 90 to 95 percent, and the purity is 99.0 to 99.9 percent.
Owner:常州佳德医药科技有限公司
Prevention or treatment agent for cerebral amyloid beta storage diseases
ActiveUS20170049744A1Inhibit progressImprove the quality of lifeOrganic active ingredientsNervous disorderAmyloid betaIguratimod
Provided is a prevention or treatment agent for cerebral amyloid beta storage diseases, that contains a substance capable of suppressing the progression, alleviating the symptoms, and improving cerebral amyloid beta storage diseases. This prevention or treatment agent for cerebral amyloid beta storage diseases has as an effective component thereof a compound (e.g., Iguratimod) indicated by formula (1) or a salt thereof and, as a result, is capable of preventing or treating cerebral amyloid beta storage diseases such as Alzheimer-type dementia or cerebral amyloid angiopathy.
Owner:TOYAMA CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com