Iguratimod crystalline form and composite thereof
A technology of crystal form and composition, applied in the field of autoimmune regulation drugs, to achieve the effect of easy production and high biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The following examples are further illustrative of the invention, not limiting.
[0080] Unless otherwise stated, herein, temperature refers to degrees Celsius (°C), and room temperature refers to about 18-23°C. The present inventors have identified several different forms of crystalline iguratimod, which have been characterized by several methods, typically XRD, DSC and IR.
[0081] According to Chem.Pharm.Bull.48131-139.2000. and Japanese Patent No97840, 4-chloro-3-nitroanisole is used as starting material to react with phenol to generate 4-phenoxy- 3-nitroanisole, iron powder reduction reaction to get 4-phenoxy-3-aminoanisole, acylation reaction with methanesulfonyl chloride to generate 3-methanesulfonamide-4-phenoxyanisole, and then Reaction with aminoacetonitrile hydrochloride, acylation, cyclization, and a total of 7 steps to prepare iguratimod. Reaction formula:
[0082]
[0083]
[0084] Alamode
[0085] Example 1. Preparation of Form 1: Take 10 grams ...
Embodiment 2
[0087] Example 2. Preparation of Form 2: Take 10 grams of Iguratimod and put it into a 1000 ml round bottom flask, add 500-1000 ml of analytically pure acetonitrile and activated carbon, heat, reflux, filter, and place the filtrate at room temperature for 24 hours, filter, filter The cake was washed and dried under reduced pressure at 50-60°C to obtain 9.5 g of Form 2. The melting point is 237-238°C.
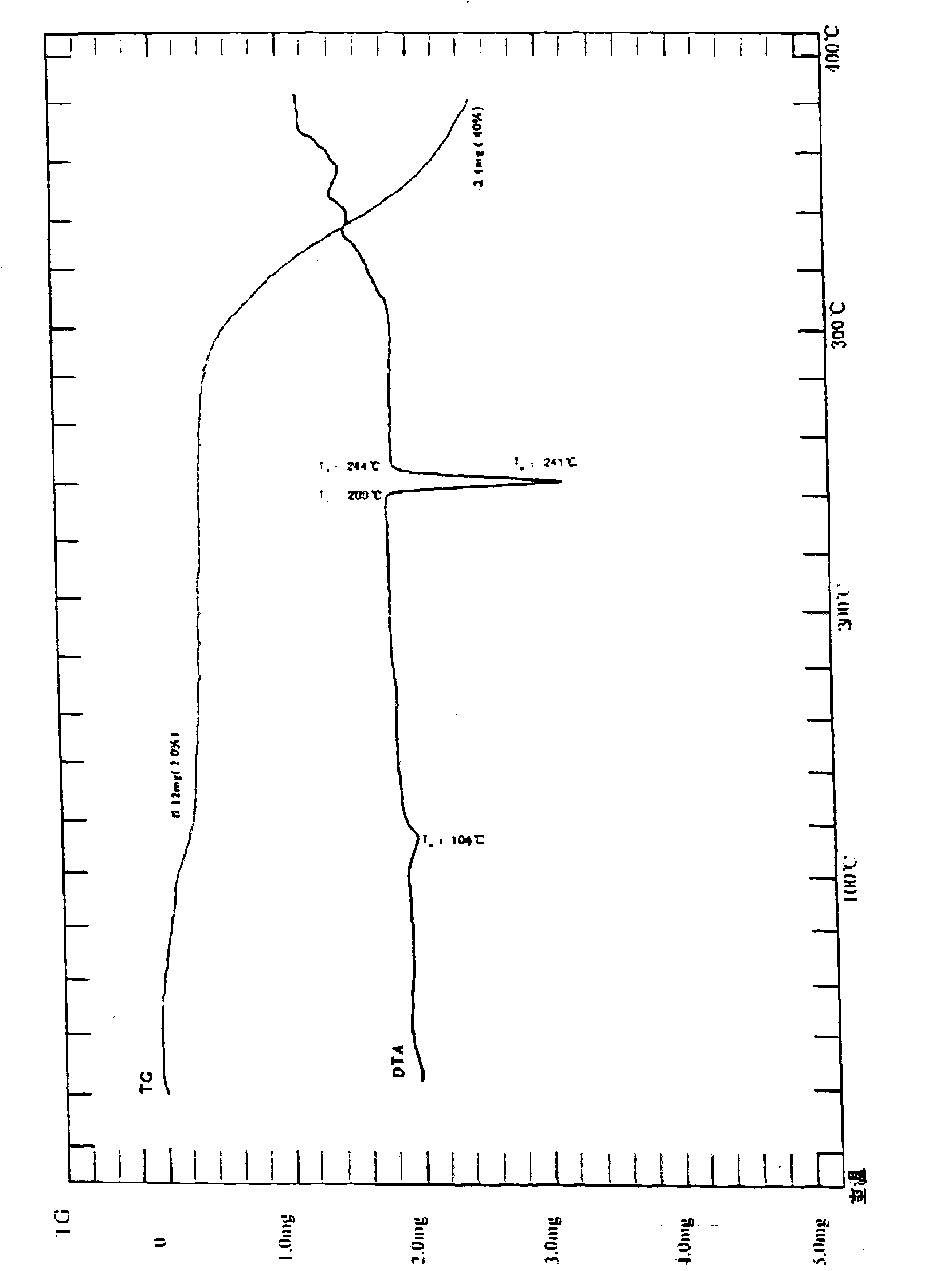
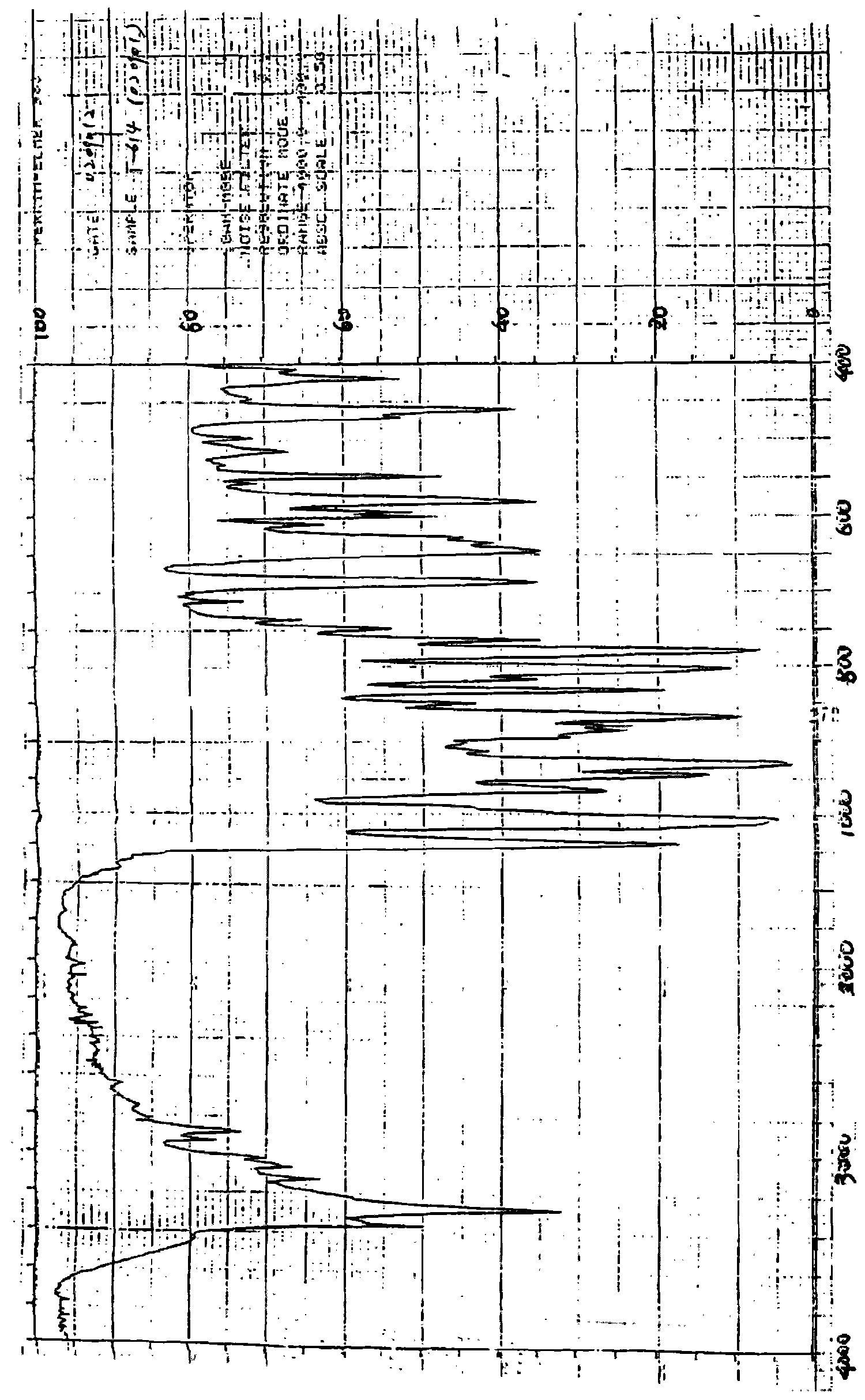
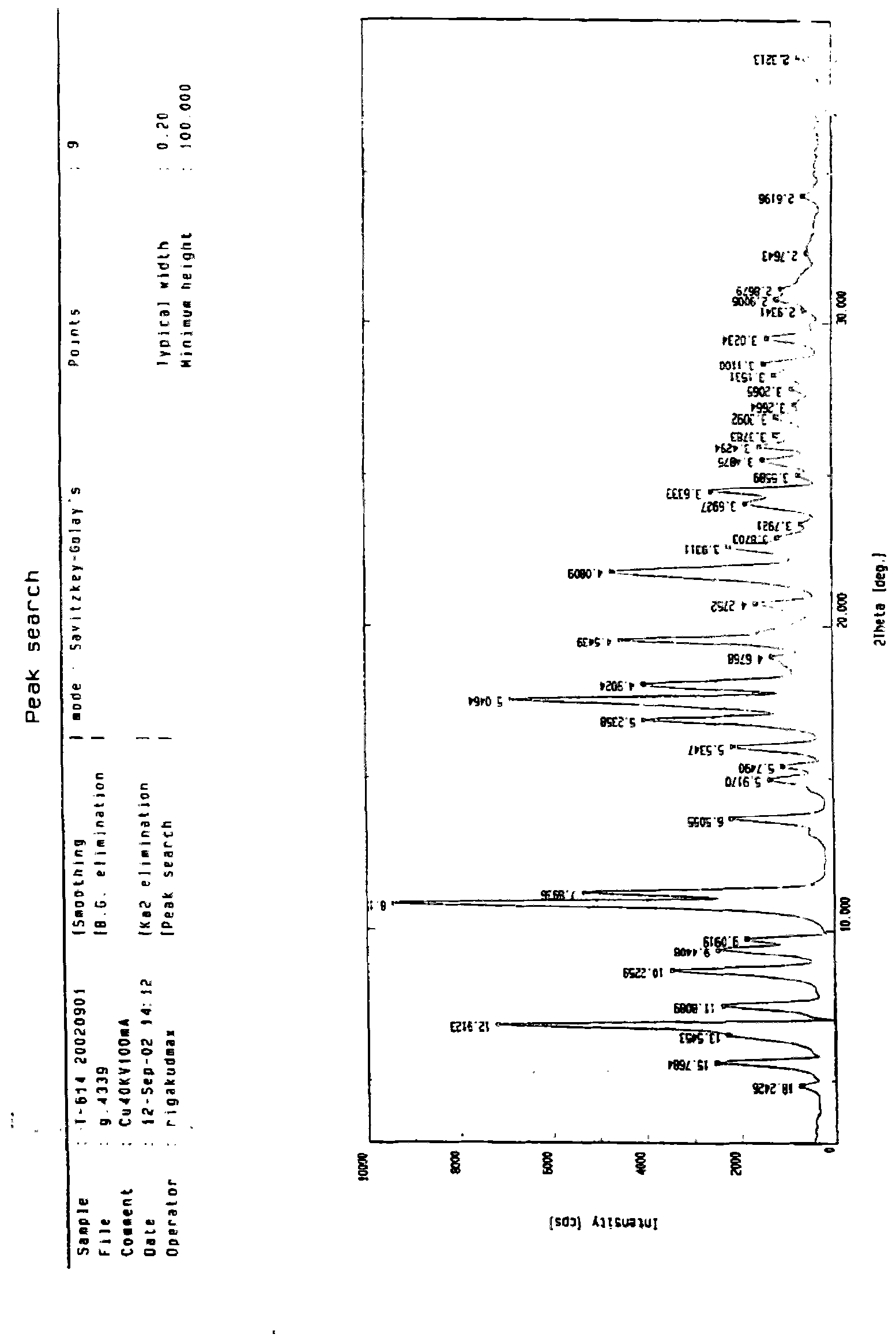
[0088] Form 2 has an XRD pattern at about 24.48, about 22.60, about 21.76, about 19.52, about 10.84, about 18.08, about 17.56, about 16.92, about 11.20, about 9.36, about 8.64, about 7.48, about 6.84, about 6.52, and about 5.60 Peak, its DSC endothermic transition is at about 236-240 ° C; the IR (KBr, cm -1 ) at about 3342, 3276, 3123, 3065, 2869, 1687, 1621, 1531, 1489, 1425, 1358, 1264 and 1156 cm -1 There are characteristic bands, differential thermogram, and infrared spectrogram are the same as Form 1, see figure 2 -3.
Embodiment 3
[0089] Example 3. Preparation of Form 3: Take 10 grams of Iguratimod and put it into a 100 ml round-bottomed flask, add 20 ml of analytically pure dimethylformamide and activated carbon, heat, reflux, and filter, and place the filtrate in a refrigerator at room temperature for 18 hours. Filter, wash the filter cake, and dry under reduced pressure at 50-60°C to obtain 9.5 g of form 3. The melting point is 237-238°C.
[0090] The XRD pattern of Form 3 has peaks at about 27.28, about 25.68, about 25.24, about 24.40, about 24.04, about 23.08, about 20.16, about 17.52, about 10.56, and about 6.00, and its DSC endothermic transition is at about 236-240°C; IR (KBr, cm -1 ) at about 3342, 3276, 3123, 3065, 2869, 1687, 1621, 1531, 1489, 1425, 1358, 1264 and 1156 cm -1 There are characteristic bands, differential thermogram, and infrared spectrogram are the same as Form 1, see figure 2 -3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com