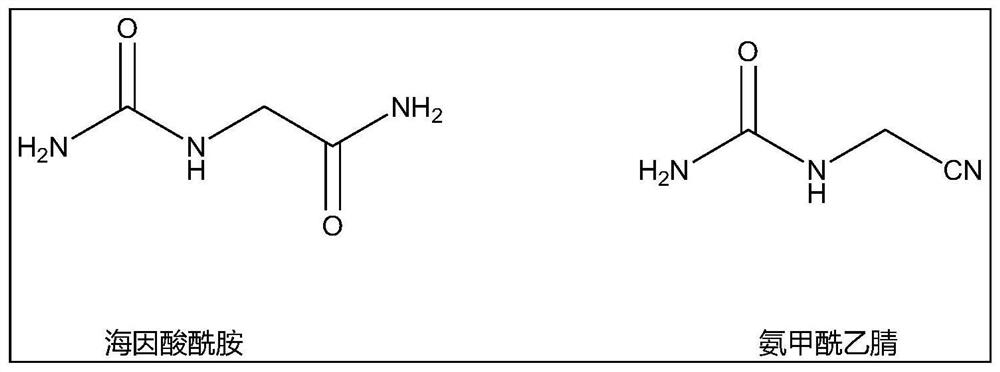
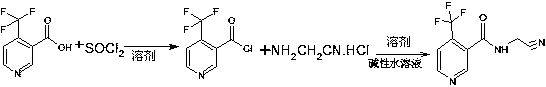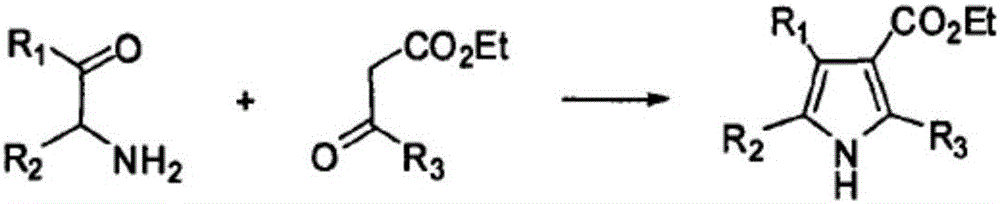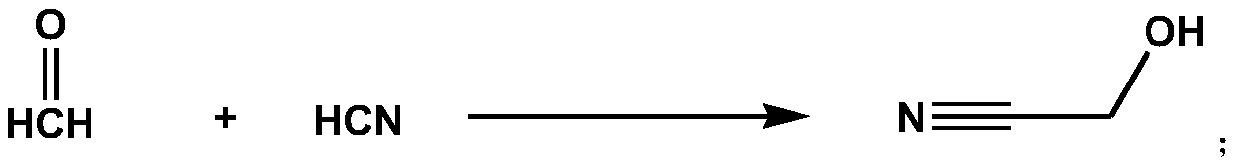Patents
Literature
61 results about "Aminoacetonitrile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aminoacetonitrile is the organic compound with the formula NCCH₂NH₂. The compound is a colorless liquid. It is unstable at room temperature, owing to the incompatibility of the amine nucleophile and the nitrile electrophile. For this reason it is usually encountered as the chloride and bisulfate salts of the ammonium derivative, i.e., [NCCH₂NH₃]⁺Cl⁻ and [NCCH₂NH₃]⁺HSO₄⁻.
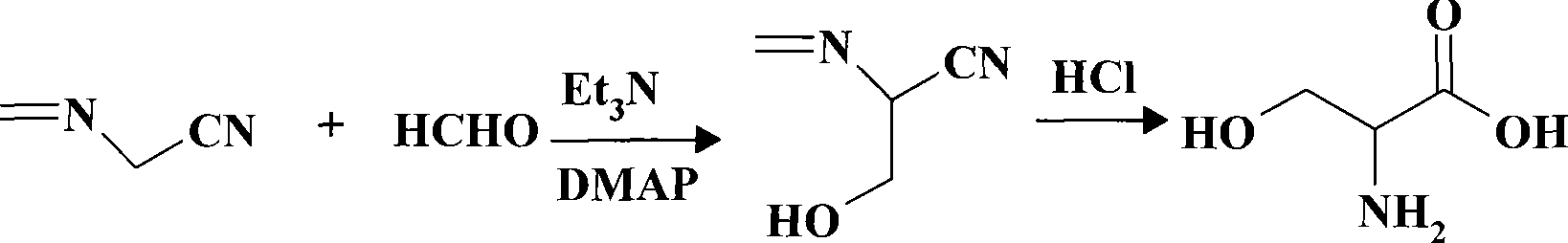
Preparation process of glycine
InactiveCN102432478AReduce pyrolysis polymerizationHigh yieldOrganic compound preparationAmino-carboxyl compound preparationReaction temperatureAlkaline hydrolysis
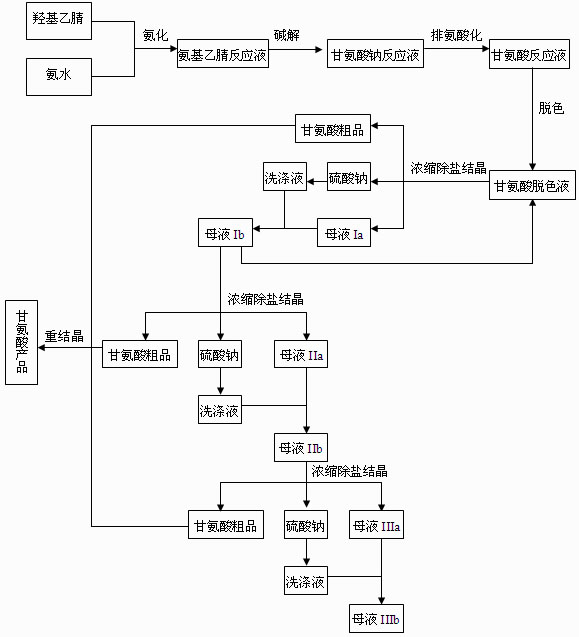
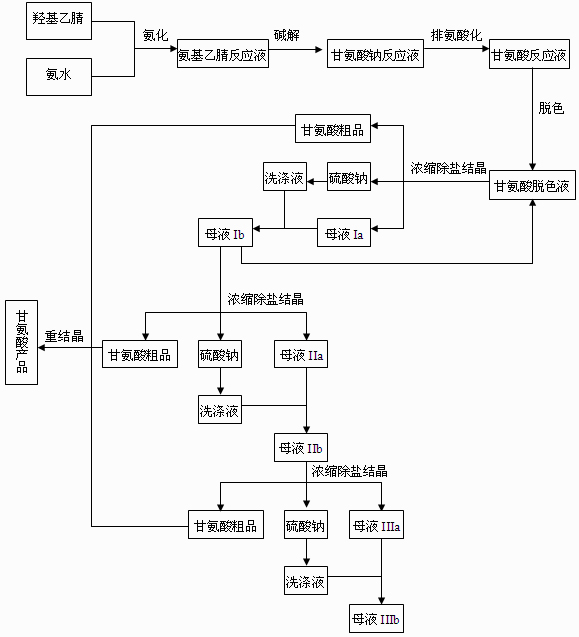
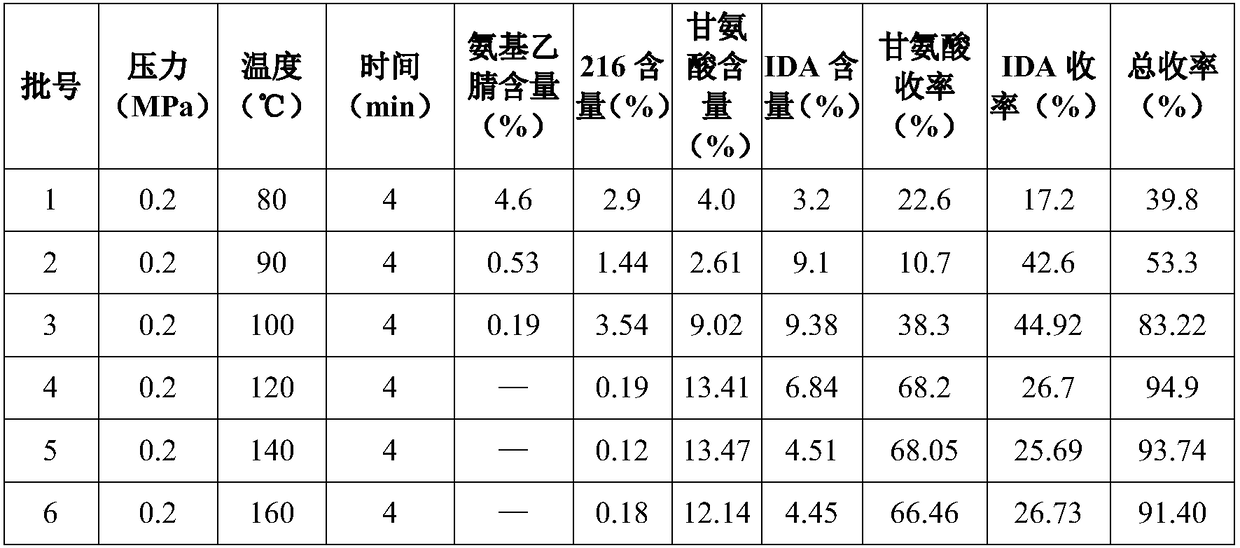
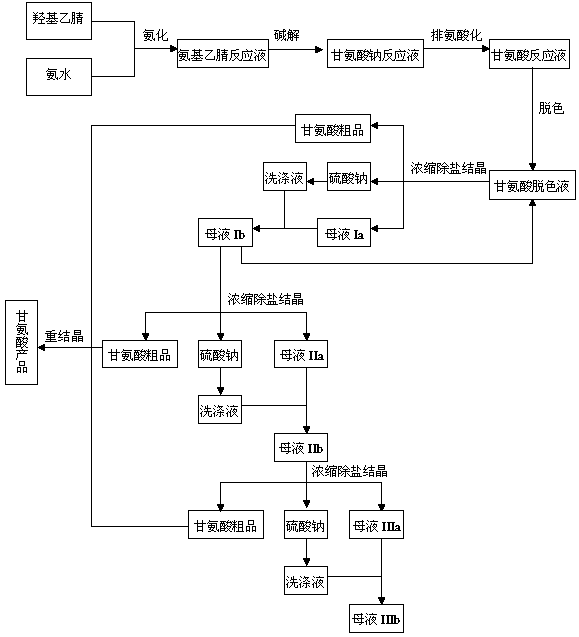
The invention discloses a preparation process of glycine, which comprises amination, alkaline hydrolysis, ammonia discharge acidification, decolorizing, concentration, desalting, crystallization and recrystallization steps, wherein in the amination step, hydroxyacetonitrile and ammonia water are mixed in a tubular reactor, and a reaction is undergone at the temperature of 50-100 DEG C under the pressure of 0.5-2.0 MPa for 4-10 minutes; and in the alkaline hydrolysis step, 30-50 percent of sodium hydroxide solution is added into an alkaline hydrolysis reactor in advance, an amination liquid is collected from an amination liquid outlet, a reaction is undergone at the temperature of 60-90 DEG C under the pressure of between -0.01 MPa and -0.09 MPa, and ammonia in a system is recovered simultaneously. In the process, the tubular reactor is taken as an amination reactor, so that the reaction temperature and pressure are raised, the reaction time is shortened, and raw material decomposition, pyrolytic polymerization of aminoacetonitrile and the generation of byproducts are reduced; alkaline hydrolysis is performed during the collection of the amination liquid, the concentration of an alkaline liquor and the alkaline hydrolysis temperature are raised simultaneously, and ammonia in the system is recovered under reduced pressure, so that the alkaline hydrolysis reaction is more complete, the speed is higher, and the generation of colored impurities is reduced; and a mother liquor circular utilizing mode is established, so that the treatment amount of waste mother liquor is reduced, the product yield is increased, and the production cost is reduced.
Owner:CHONGQING UNISPLENDOUR CHEM
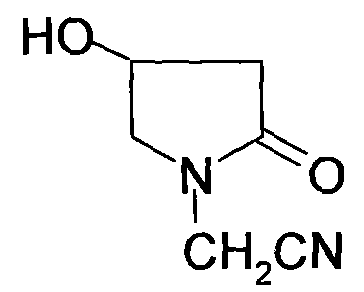
Method for preparing 4-hydroxylethylpyrrolidone-2-acetamide
InactiveCN101693685ARaw materials are cheap and easy to getLow costOrganic chemistryAcetoacetatesAcetic acid
Owner:SUZHOU HOPE TECH
Solid sodium sarcosine preparation method
PendingCN103664665ALower requirementHigh purityOrganic compound preparationAmino-carboxyl compound preparationSodium methoxideSeparation technology
The invention discloses a high-purity solid sodium sarcosine preparation method. The specific technical scheme comprises the following steps: condensing hydroxyacetonitrile and methylamine to obtain methylaminoacetonitrile; adding sodium hydroxide, and hydrolyzing to obtain sodium methylaminoacetate, namely a sodium sarcosine liquid and free sodium hydroxide mixture; adding hydrochloric acid which is equal to the sodium sarcosine in mol, and neutralizing to obtain a sarcosine and sodium chloride mixture; separating sodium chloride and sarcosine through an electrodialysis membrane separation technology to obtain a high-purity sarcosine solution and an impurity solution; distilling the high-purity sarcosine solution with a multifunctional evaporator to obtain solid crystals, separating to obtain pure sarcosine, and dissolving the sarcosine with 0.5 time of purified water; and dropwisely adding 1:0.98 mol of sodium methoxide at 40 DEG C to precipitate solid sodium sarcosine, performing centrifugal separation to obtain white solids, and drying to obtain the finished product, wherein after the cycle is completed, the mother solution can be recovered through rectifying, and methanol can be for private use or can be sold as a byproduct. The process has peculiar characteristics; and high-purity sodium sarcosine solids can be prepared through the process.
Owner:TIANJIN TIANCHENG PHARMA
Method for producing ethylenediamine
InactiveUS7915454B2Speed up the conversion processSimple and inexpensive and variableOrganic compound preparationAmino compound preparationEthylenediamineHydrogen
The invention relates to a process for preparing ethylenediamine by hydrogenation of aminoacetonitrile over a catalyst, wherein the hydrogenation is carried out in a solution comprising aminoacetonitrile, water in a proportion of from 0 to 60% by weight and a solvent and the aminoacetonitrile comprised in the solution is fed into the reaction vessel at a rate which is not greater than the rate at which the aminoacetonitrile reacts with hydrogen in the hydrogenation.
Owner:BASF AG
Synthesis method of alkylglycine
PendingCN108358797AHigh yieldHigh purityCarboxylic acid nitrile preparationOrganic compound preparationHigh volume manufacturingAminoacetonitrile
The invention discloses a synthesis method of alkylglycine. According to the method, N-(diphenylmethylene)aminoacetonitrile is used as a raw material to take a substitution reaction with 2-alkyl halide under the effect of a catalyst of lithium diisopropylamide so as to synthesize a fire-new intermediate compound of (3S)-2-((diphenylmethylene)amino)-3-methylalkyl nitrile. The method can be appliedto the synthesis of alkylglycine such as isoamylglycine, isohexyl glycine and isoheptyl glycine; products can be used as necessary intermediates for pesticides and medicine. By using the synthesis method provided by the invention, the raw material price is low; the production cost is low; the product purity is high; the reaction steps are simple; the reaction speed is high; the conditions are mild; the reaction treatment is simple and convenient; the method is more suitable for mass production.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for synthesizing glycine by performing alkaline hydrolysis on aminoacetonitrile continuously and rapidly
ActiveCN108623489AShorten the alkaline hydrolysis timeReduce pyrolysis polymerizationOrganic compound preparationChemical/physical/physico-chemical microreactorsGlycineReaction rate
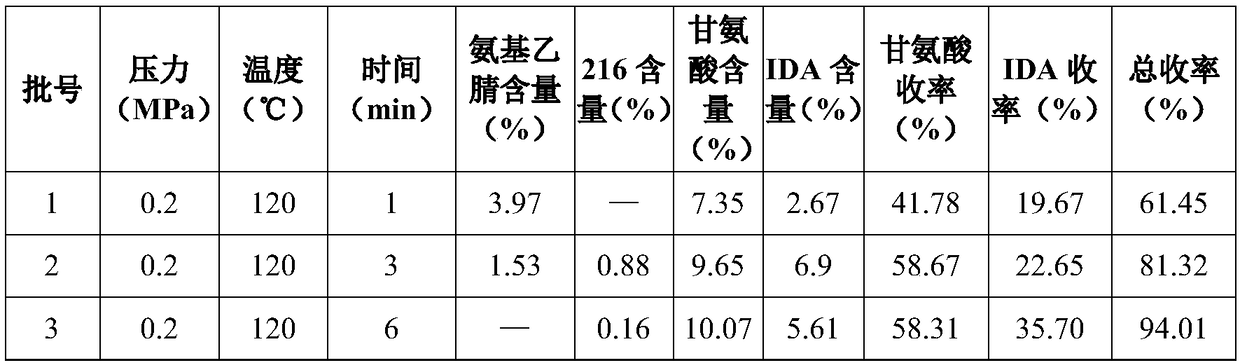
The invention provides a method for synthesizing glycine by performing alkaline hydrolysis on aminoacetonitrile continuously and rapidly. The method comprises the following steps: adding alkali and aminoacetonitrile into a microchannel reactor, controlling the reaction temperature to 100 to 160 DEG C and the pressure to 0.1 to 0.5 MPa and enabling the retention time of the reactants in the microchannel to be 1 to 10 minutes to obtain the glycine. The microchannel reactor is adopted, so that the alkaline hydrolysis time of the aminoacetonitrile is greatly shortened, and pyrolysis olymerizationof the aminoacetonitrile and generation of byproducts are reduced; and the alkaline hydrolysis reaction rate is increased, the yield of products is increased and the production cost is reduced.
Owner:重庆紫光川庆化工有限责任公司
Preparation process of glycine
InactiveCN102432478BReduce pyrolysis polymerizationHigh yieldOrganic compound preparationAmino-carboxyl compound preparationReaction temperatureAlkaline hydrolysis
The invention discloses a preparation process of glycine, which comprises amination, alkaline hydrolysis, ammonia discharge acidification, decolorizing, concentration, desalting, crystallization and recrystallization steps, wherein in the amination step, hydroxyacetonitrile and ammonia water are mixed in a tubular reactor, and a reaction is undergone at the temperature of 50-100 DEG C under the pressure of 0.5-2.0 MPa for 4-10 minutes; and in the alkaline hydrolysis step, 30-50 percent of sodium hydroxide solution is added into an alkaline hydrolysis reactor in advance, an amination liquid is collected from an amination liquid outlet, a reaction is undergone at the temperature of 60-90 DEG C under the pressure of between -0.01 MPa and -0.09 MPa, and ammonia in a system is recovered simultaneously. In the process, the tubular reactor is taken as an amination reactor, so that the reaction temperature and pressure are raised, the reaction time is shortened, and raw material decomposition, pyrolytic polymerization of aminoacetonitrile and the generation of byproducts are reduced; alkaline hydrolysis is performed during the collection of the amination liquid, the concentration of an alkaline liquor and the alkaline hydrolysis temperature are raised simultaneously, and ammonia in the system is recovered under reduced pressure, so that the alkaline hydrolysis reaction is more complete, the speed is higher, and the generation of colored impurities is reduced; and a mother liquor circular utilizing mode is established, so that the treatment amount of waste mother liquor is reduced, the product yield is increased, and the production cost is reduced.
Owner:CHONGQING UNISPLENDOUR CHEM
Method for preparing aminoacetonitrile and N,N-dimethylcyanamide from methane and ammonia gas through plasma synthesis
InactiveCN104725271ASimple methodNo pollution in the processOrganic compound preparationPreparation by hydrocarbon ammoxidationEthylenediamineAlkane
The invention relates to a method for synthesizing aminoacetonitrile and N,N-dimethylcyanamide by using methane and ammonia gas as raw materials, which is characterized by comprising the following steps: performing non-equilibrium plasma activation on methane and ammonia gas to generate carbon-containing and nitrogen-containing free radical active species, and performing one-step spontaneous reaction on the species to generate the target products. The method belongs to a one-step direct synthesis process, has the advantages of simplicity and cheap raw materials, does not use solvent and causes no pollution. Besides, the method is also applicable to synthesis of organic compounds from various C1-5 alkane, olefin and alkyne and ammonia gas. Moreover, in addition to the aminoacetonitrile and N,N-dimethylcyanamide, acetonitrile, ethylenediamine, 4,5-dihydro-5-methylpyrazole, aminopyrazole and other products can be also obtained through the plasma synthesis method.
Owner:DALIAN UNIV OF TECH
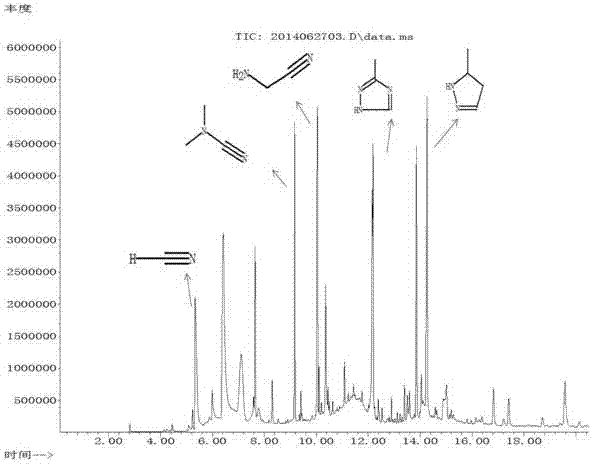
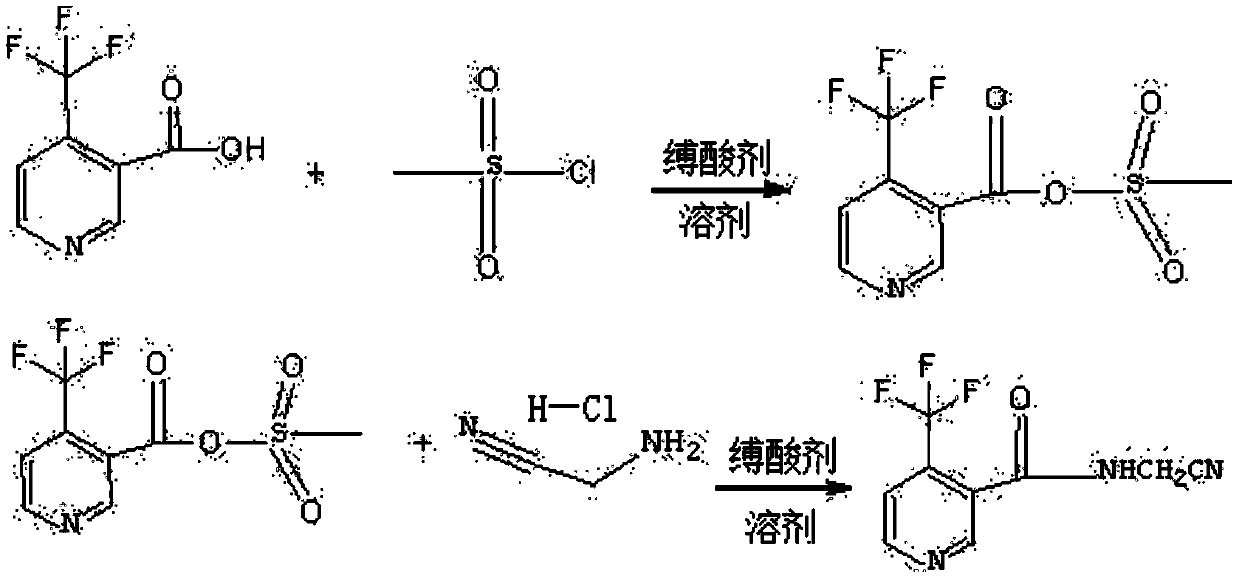
Synthesis method of N-cyanomethyl-4-(trifluoromethyl)-nicotinamide
PendingCN109851552AImprove conversion rateHigh purityOrganic chemistrySulfonyl chlorideSynthesis methods
The invention discloses a synthesis method of N-cyanomethyl-4-(trifluoromethyl)-nicotinamide, and belongs to the technical field of pesticides. According to the method, 4-trifluoromethylnicotinic acidand methane sulfonyl chloride are taken as the primary raw materials and carry out reactions in the presence of an acid binding agent and a solvent to prepare acid anhydrides; then in the presence ofan acid binding agent, acid anhydrides and amino acetonitrile hydrochloride carry out reactions to prepare N-cyanomethyl-4-(trifluoromethyl)-nicotinamide. 4-trifluoromethylnicotinic acid and methanesulfonyl chloride are taken as the primary raw materials to synthesize acid anhydrides at first, acid anhydrides are resistant to hydrolysis, then acid anhydrides react with amino acetonitrile hydrochloride to prepare N-cyanomethyl-4-(trifluoromethyl)-nicotinamide; the conversion rate is high (not less than 96%); the purity is high, and the yield is good (not less than 90%). Toxic phosgene is notused, the safety is greatly improved, moreover, the operation is simple, and the synthesis method is suitable for industrial production.
Owner:JINGBO AGROCHEM TECH CO LTD
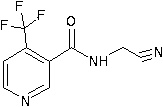
N-cyanomethyl-4-(trifluoromethyl) nicotinamide preparation method
The invention belongs to the technical field of pesticide synthesis, in particular to an N-cyanomethyl-4-(trifluoromethyl) nicotinamide preparation method. The method includes the following steps: reacting, by 4-(trifluoromethyl) niacinamide, with thionyl chloride to obtain acyl chloride; and then reacting with aminoacetonitrile hydrochloride to synthesize the N--cyanomethyl-4-(trifluoromethyl) nicotinamide under an inorganic weak alkaline aqueous solution environment. According to the invention, the preparation method is simple in process, simple in operation, high in yield coefficient (be equal or greater than 92% of mole yield coefficient in terms of trifluoromethylnicotinamide), few in impurities, the purity can reach be equal or greater than 99. 5%, low in production costs, convenientfor solvent recovery, recyclable, less in pollution, high in safety, and suitable for industrial production.
Owner:山东沾化永浩医药科技有限公司
Method for synthesizing methenamine and N,N-dimethylcyanamide
The present invention provides a method for synthesizing nitrogen-containing organic matters with high added values by virtue of a one-step method by activating methanol and ammonia gas molecules by means of a non-equilibrium plasma technology, namely a method for synthesizing methenamine and N,N-dimethylcyanamide. The method provided by the present invention has the advantages of low price of reaction raw materials and abundant sources, and belongs to one-step method synthesis. Moreover, the method provided by the invention has the advantages of being environmental-friendly, mild in condition, convenient to operate, simple in process, high in adaptability and the like, and can be used for synthesizing ethanol, formamide, ethanamide, aminoacetonitrile, N,N-dimethylcyanamide, glycol and 1H-1,2,4-triazole by further changing the condition. In addition, the method is suitable for synthesizing the nitrogen-containing compounds with high added values by any one or two of methanol, ethanol and propanol and the ammonia under the condition of non-equilibrium plasmas.
Owner:DALIAN UNIV OF TECH
Preparation method of aminoacetonitrile hydrochloride
InactiveCN102432501AHigh yieldSimple and fast operationCarboxylic acid nitrile preparationOrganic compound preparationAminoacetonitrileAcetonitriles
The invention relates to a preparation method of aminoacetonitrile hydrochloride, which comprises the following steps: (1) carrying out condensation reaction by using ammonium chloride, formaldehyde, acetic acid and sodium cyanide as reaction raw materials to generate aminoacetonitrile: adding the ammonium chloride, formaldehyde and water into a reactor, uniformly stirring, cooling to below 0 DEG C, dropwisely adding 30-40 wt% sodium cyanide aqueous solution, starting to dropwisely add the acetic acid when 40-60% sodium cyanide aqueous solution is dropwisely added in the reactor, continuing the reaction at below 0 DEG C for 1-2 hours, filtering, and centrifugalizing to obtain the aminoacetonitrile; and (2) mixing the aminoacetonitrile with hydrogen chloride methanol solution, reacting at 45-50 DEG C for 1-2 hours, cooling to below 5 DEG C, filtering, and centrifugalizing to obtain the aminoacetonitrile hydrochloride, wherein in the hydrogen chloride methanol solution, the content of hydrogen chloride is 30-50 wt%, and the water content is less than or equal to 1%. According to the method provided by the invention, the total yield of the target product is high, and the operation is simple.
Owner:太仓市茜泾化工有限公司
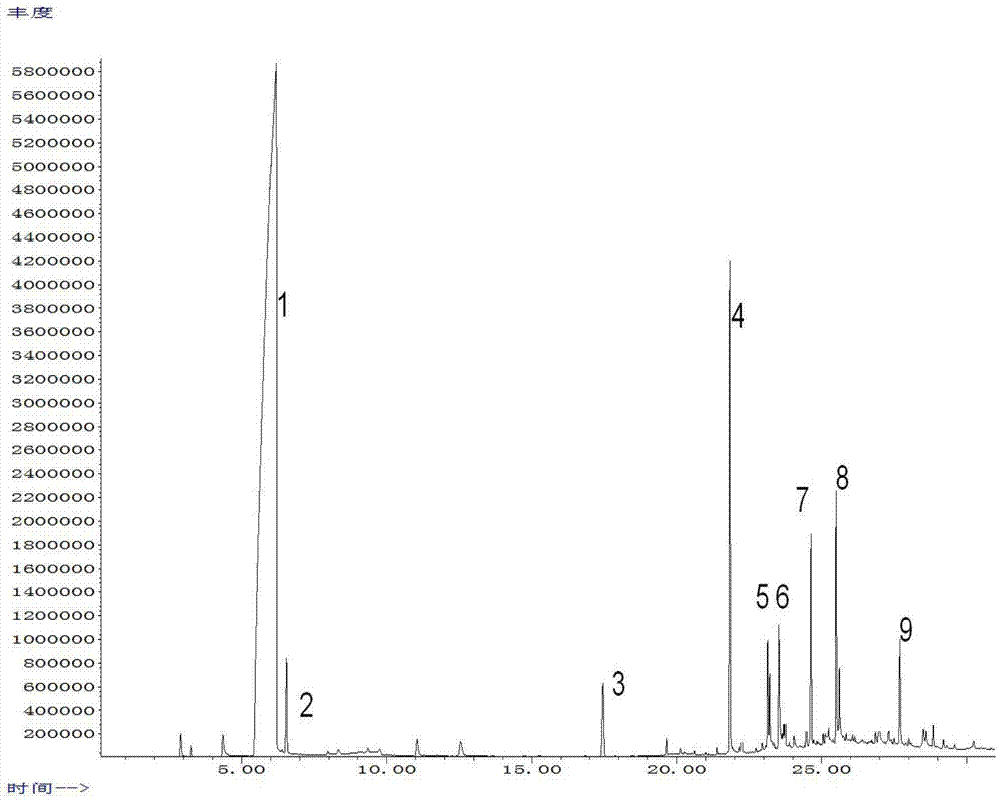
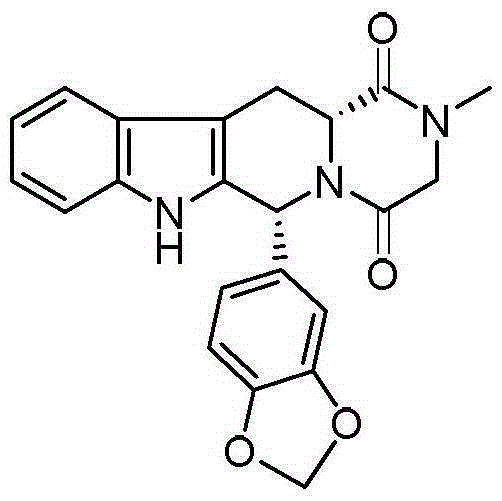
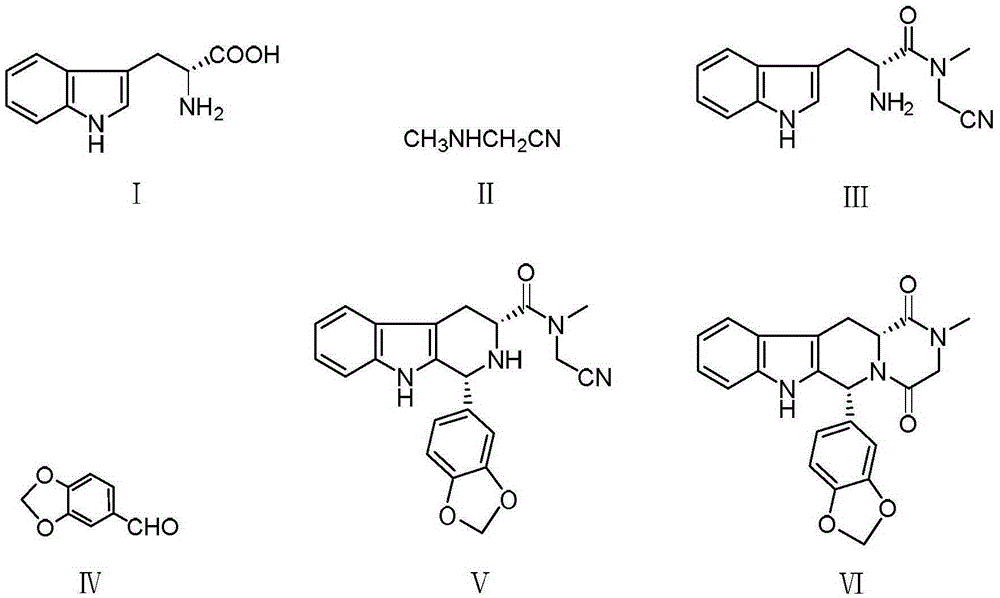
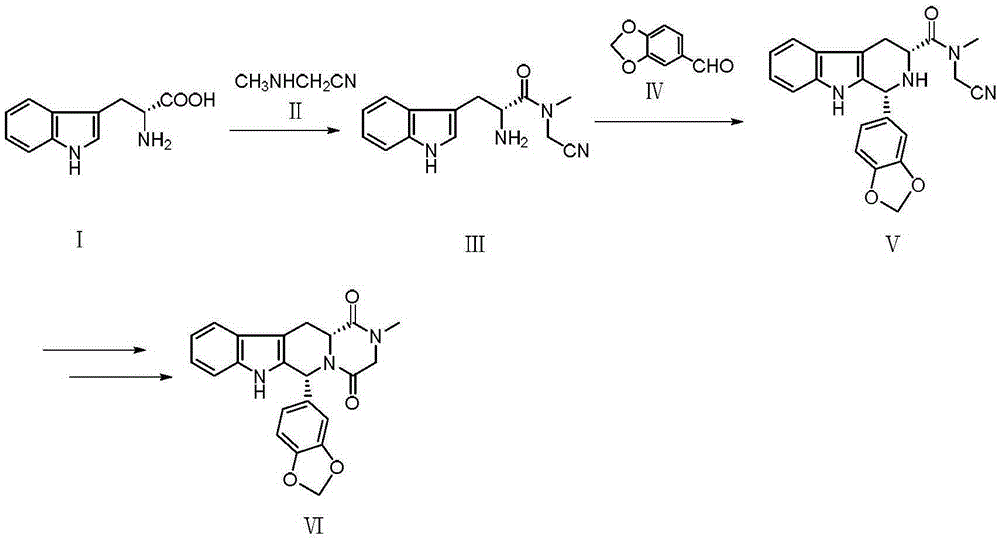
Novel synthetic method for tadalafil
The invention relates to a synthetic method for a compound in the technical field of medicine and chemical engineering, and particularly relates to a novel synthetic method for tadalafil. The synthetic method comprises the steps of: 1) condensing an initial raw material which is D-tryptophan (I) and methylamino acetonitrile (II) or salt thereof to obtain a compound as shown in a formula III; 2) carrying out Pictet-Spengler cyclization reaction on the compound as shown in the formula III and heliotropin and carrying out crystallization to obtain a compound as shown in a formula V; and 3) hydrolyzing the compound V under an acidic or alkaline condition by cyano and then condensing the compound to obtain the target product tadalafil (VI). The formula is shown in the description. In the synthetic method provided by the invention, chemical raw materials which are great in toxicity, severe in environmental pollution and flammable and combustible such as thionyl chloride, chloroacetyl chloride, methylamine and the like. The novel synthetic method for tadalafil provided by the invention is simple in reaction method, convenient to operate and high in yield.
Owner:ZHEJIANG YONGNING PHARMA
Method for preparing aminoacetonitrile sulfate
ActiveCN102531960AHigh yieldSimple and fast operationCarboxylic acid nitrile preparationOrganic compound preparationAcetic acidSulfate
The invention relates to a method for preparing aminoacetonitrile sulfate. The method comprises the following steps of: (1) performing condensation on ammonium chloride, formaldehyde, acetic acid and sodium cyanide which are taken as reaction raw materials to obtain aminoacetonitrile, namely putting ammonium chloride, formaldehyde and water into a reactor, stirring uniformly, cooling to below zero, dripping 30 to 40 weight percent of aqueous solution of sodium cyanide, simultaneously dripping the acetic acid when 40 to 60 percent of aqueous solution of sodium cyanide is dripped, continuing to react below zero for 1 to 2 hours after the dripping is finished, filtering, and centrifuging to obtain the aminoacetonitrile; and (2) reacting the aminoacetonitrile and a methanol solution of sulfuric acid at the temperature of between 30 and 35 DEG C for 1 to 2 hours, cooling to below 5 DEG C, filtering, and centrifuging to obtain the aminoacetonitrile sulfate, wherein the sulfuric acid accounts for less than or equal to 15 percent of the weight of the methanol solution of the sulfuric acid and the water accounts for less than or equal to 1 percent of the weight of the methanol solution of the sulfuric acid. According to the method, the total yield of a target product is high; and the method is easy to operate.
Owner:太仓市茜泾化工有限公司
Method for producing ethyleneamines
InactiveUS7880035B2Speed up the conversion processSimple and inexpensiveCarboxylic acid nitrile preparationOrganic compound preparationEthylenediamineDiethylenetriamine
The invention relates to a process for preparing an ethylene amine mixture, which comprises hydrogenating an amino nitrile mixture comprising at least 30% by weight of aminoacetonitrile (AAN) and at least 5% by weight of iminodiacetonitrile (IDAN) in the presence of a catalyst. Ethylenediamine (EDA) and / or diethylenetriamine (DETA) and, if appropriate, further ethylene amines can be isolated from the ethylene amine mixtures obtained.
Owner:BASF AG
Method for preparing methylamino-acetonitrilehydrochlorate
ActiveCN101402588AAvoid hydrolysisCarboxylic acid nitrile preparationOrganic compound preparationPropanoic acidAcetonitrile
The invention relates to a preparation method for methylamino-acetonitrile hydrochlorate. The preparation method comprises the following steps: (1) using methylamine hydrochlorate, sodium cyanide and formaldehyde as reaction raw materials, and performing reaction in the presence of a catalyst to generate methylamino-acetonitrile; (2) causing the methylamino-acetonitrile to react with hydrochloric acid to generate the methylamino-acetonitrile hydrochlorate, wherein in step (1), below zero DEG C, using 3-hydrosulfuryl propanoic acid as a catalyst with the dose of 0.9 to 1.1 times of the sodium cyanide. The preparation method not only has simple operation, but also has high yield and low cost, and is suitable for industrial production.
Owner:太仓市茜泾化工有限公司
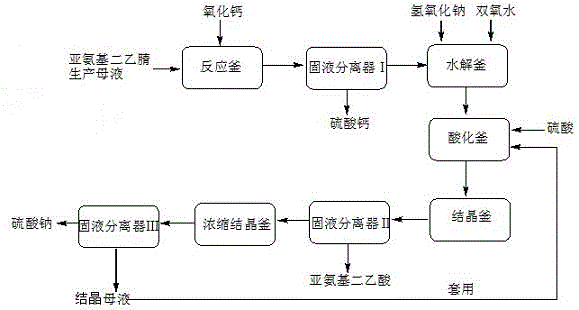
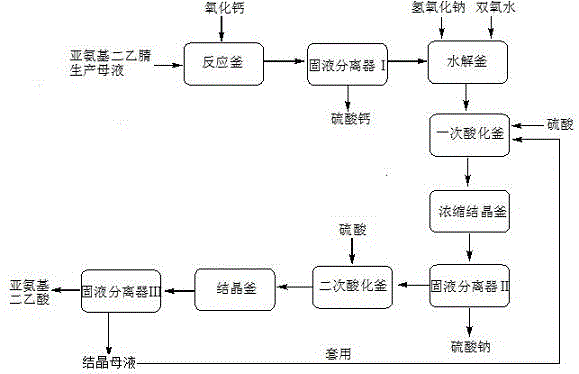
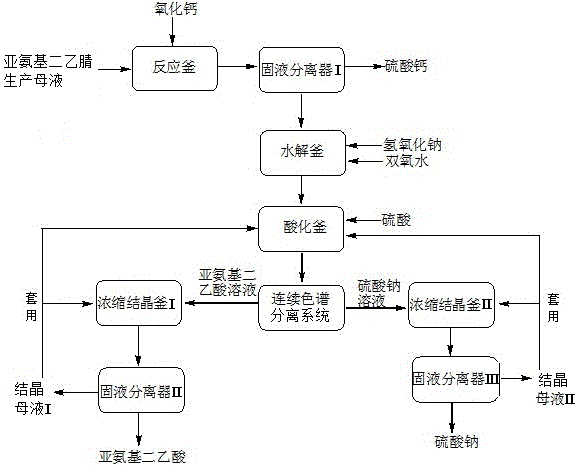
Method and apparatus for preparing iminodiacetic acid by using iminodiacetonitrile to produce mother liquor
InactiveCN104974054AEfficient recyclingAvoid secondary pollutionOrganic compound preparationAmino-carboxyl compound preparationSodium sulfateSodium salt
The invention discloses a method and apparatus for preparing an iminodiacetic acid by using iminodiacetonitrile to produce mother liquor. The method comprises: adding calcium oxide or calcium hydroxide to the mother liquor to react so that glycolonitrile and aminoacetonitrile contained in the mother liquor are transformed into minodiacetonitrile, transforming sulphuric acid, ammonium and ammonium sulfate into calcium sulfate, and thus obtaining solid calcium sulphate and iminodiacetonitrile solution through solid-liquid separation; and hydrolyzing the iminodiacetonitrile solution into iminodiacetic acid disodium salt by using sodium hydroxide, using peroxide to decolorize the iminodiacetic acid disodium salt, performing the separation of a destaining solution by using the sulfuric acid to acidify once and then perform multiple crystallization or firstly performing continuous chromatography and then performing crystallization separation separately, or using the sulfuric acid to perform graded acidification, and then performing crystallization separation separately so as to obtain solid iminodiacetic acid and solid sodium sulfate. The method of the invention is simple in process and low in investment; not only iminodiacetonitrile produced mother liquor is effectively treated, an expensive incinerator is not needed to be bought, and secondary pollution of waste gas from an incinerator can also be prevented; furthermore, the glycolonitrile, aminoacetonitrile and iminodiacetonitrile contained in the mother liquor can be effectively recovered so as to prepare iminodiacetic acid with economic value, thereby reducing the production cost.
Owner:CHONGQING UNISPLENDOUR CHEM
Synthesis method of flonicamid
The invention discloses a synthesis method of flonicamid, and belongs to the technical field of organic synthesis. 4-trifluoromethyl nicotinic acid and aminoacetonitrile hydrochloride are subjected to a reflux reaction in a dichloromethane and water mixed system in the presence of a mixed catalytic system, after water is added for crystallization, filtration and drying are performed to obtain a white crystal flonicamid product, the purity can reach 99.0% or above, and the white crystal flonicamid product is dried, sealed and stored. The method is simple in route operation, few in reaction by-products, high in product purity, low in cost and high in market competitiveness.
Owner:HUBEI JINGHONG CHEM
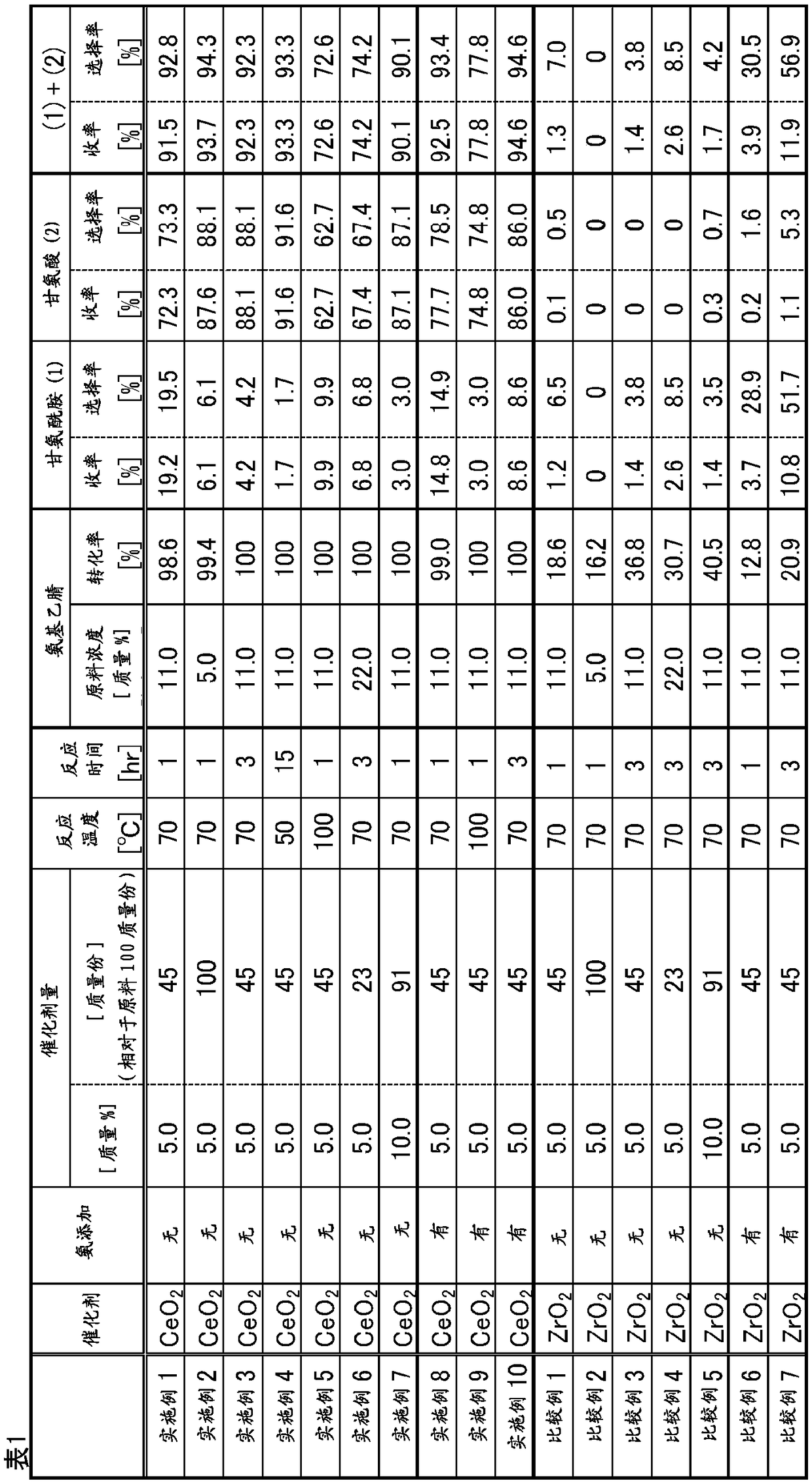
Method for producing glycine
ActiveCN109415299AHigh yieldHigh activityOrganic compound preparationOrganic chemistry methodsGlycinonitrileAminoacetonitrile
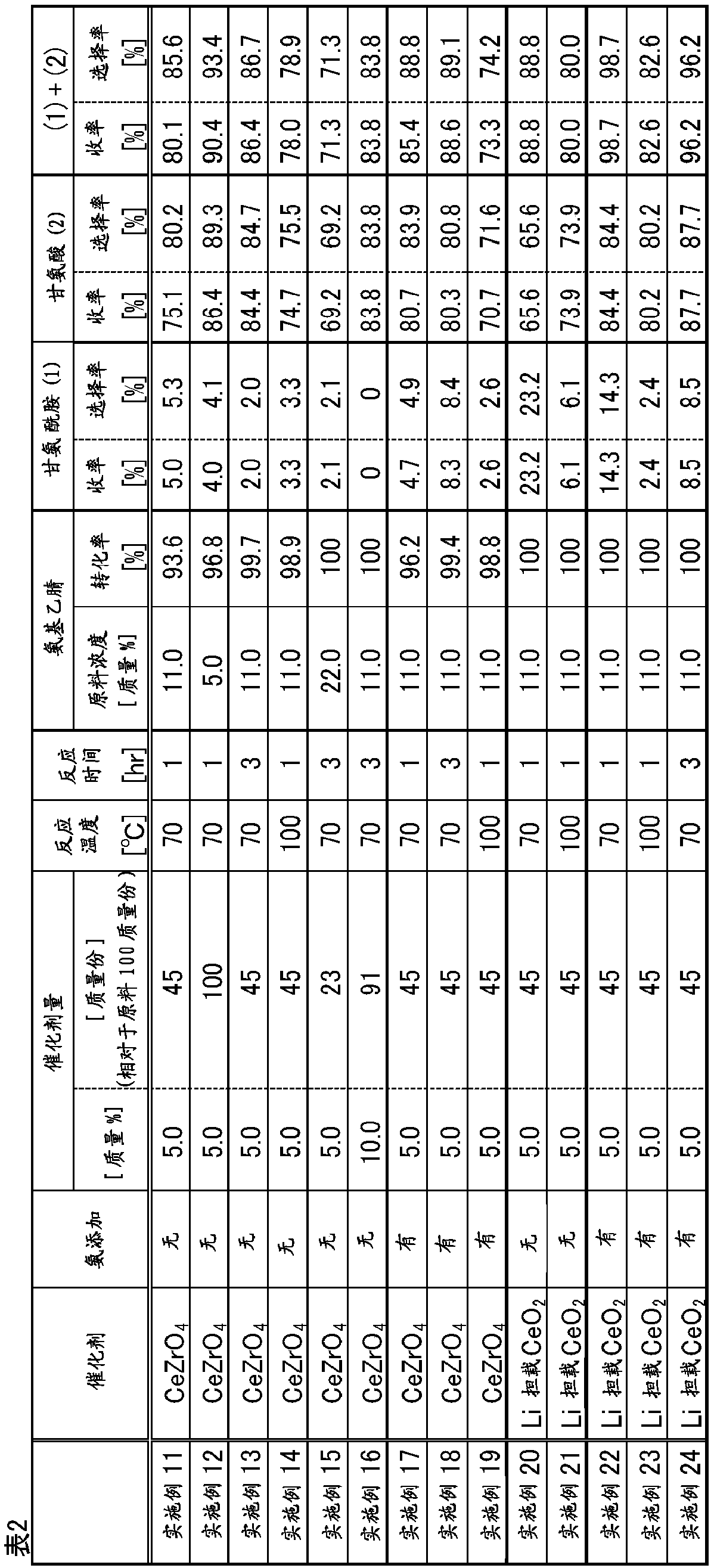
Provided is a method for producing glycine with which it is possible to obtain glycine at a higher yield than with conventional methods, when glycine is synthesized from glycinonitrile. The method forproducing glycine is characterized in that glycine is obtained by reacting, in the presence of a cerium compound, glycinonitrile and water after further adding ammonia.
Owner:RESONAC CORP
Method for producing aminonitriles
InactiveUS8153845B2Speed up the processIsocyanic acid derivatives preparationCarboxylic acid nitrile preparationAminoacetonitrileCyanohydrin
The invention relates to a process for preparing an amino nitrile mixture comprising aminoacetonitrile (AAN) and from 5 to 70% by weight of iminodiacetonitrile (IDAN), which comprises heating crude AAN which is largely free of formaldehyde cyanohydrin (FACH-free) at a temperature of from 50 to 150° C.
Owner:BASF AG
Industrialized compounding method of N-alkyl substituted-5-cyanoimidazole compound
InactiveCN101550105AReaction is easy to controlReduce manufacturing costOrganic chemistryState of artChemical reaction
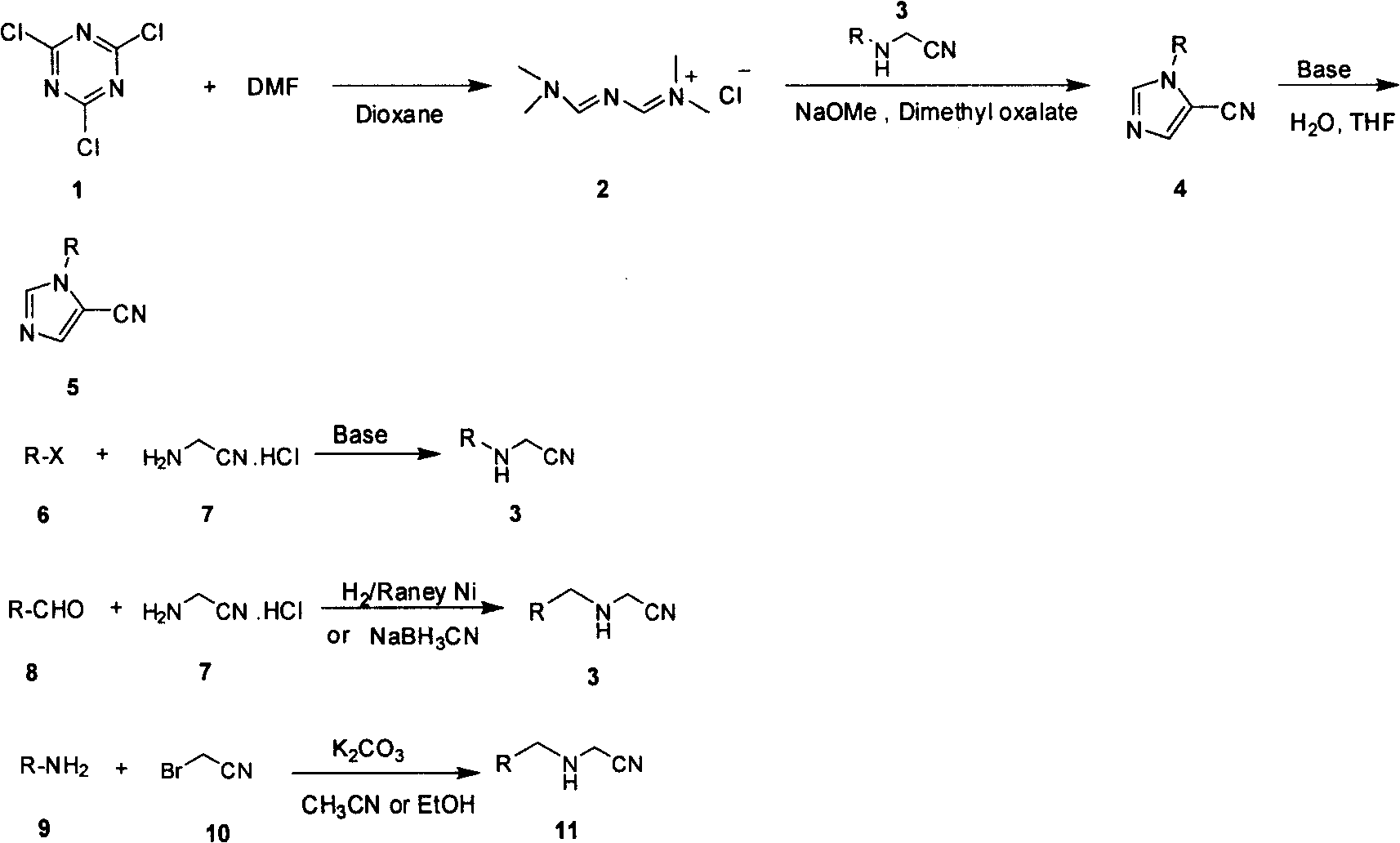
The invention relates to an industrialized compounding method of an N-alkyl substituted-5-cyanoimidazole compound, which uses conventional and easily-obtained cyanuric chloride as the raw material to react with DMF to generate a gold reagent and react with alkyl-substituted aminoacetonitriles to obtain the N-alkyl substituted-5-cyanoimidazole compound under an alkali condition in a loop closure way. The chemical reaction formula is as the right formula. The invention solves the problems of long line, low yield, difficult purification, no effective compounding method and no scale production of the prior art and can realize the scale industrialized production.
Owner:上海药明康德新药开发有限公司 +1
A method for synthesizing aminoacetonitrile and n,n-dimethylcyanamide with methane and ammonia plasma
InactiveCN104725271BSimple methodNo pollution in the processOrganic compound preparationPreparation by hydrocarbon ammoxidationEthylenediamineAlkane
The invention relates to a method for synthesizing aminoacetonitrile and N,N-dimethylcyanamide by using methane and ammonia gas as raw materials, which is characterized by comprising the following steps: performing non-equilibrium plasma activation on methane and ammonia gas to generate carbon-containing and nitrogen-containing free radical active species, and performing one-step spontaneous reaction on the species to generate the target products. The method belongs to a one-step direct synthesis process, has the advantages of simplicity and cheap raw materials, does not use solvent and causes no pollution. Besides, the method is also applicable to synthesis of organic compounds from various C1-5 alkane, olefin and alkyne and ammonia gas. Moreover, in addition to the aminoacetonitrile and N,N-dimethylcyanamide, acetonitrile, ethylenediamine, 4,5-dihydro-5-methylpyrazole, aminopyrazole and other products can be also obtained through the plasma synthesis method.
Owner:DALIAN UNIV OF TECH
Synthesis method of 1-amino-1-cyclopropanecarbonitrile hydrochloride
InactiveCN107652207AMild reaction conditionsHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationSulfonyl chlorideSynthesis methods
The invention discloses a synthesis method of 1-amino-1-cyclopropanecarbonitrile hydrochloride. The method includes: using aminoacetonitrile as the starting material to react with benzene sulfonyl chloride to obtain N, N-dibenzenesulfonyl acetonitrile, further carrying out ring closing reaction with 1, 2-dibromoethane under a sodium hydride alkaline condition to obtain N, N- benzenesulfonamidocyclopropanecarbonitrile, and finally conducting deprotection of benzoyl under an acidic condition so as to obtain 1-amino-1-cyclopropanecarbonitrile hydrochloride. The method provided by the invention has the advantages of mild reaction conditions, high yield and low cost, and is suitable for industrial application.
Owner:JIANGSU CANCER HOSPITAL
Glycine preparation method for reducing iminodiacetic acid content
PendingCN114524738AAvoid generatingAdd lessCarboxylic acid nitrile preparationOrganic compound preparationHydantoic acidHydrolysis
The invention relates to the technical field of organic chemical industry, in particular to a glycine preparation method for reducing the content of iminodiacetic acid, which comprises the following steps: adding a carbon source stabilizer in the ammoniation process of hydroxyacetonitrile and an ammonia source, reacting to obtain a glycine nitrile stabilizing solution, and hydrolyzing the glycine nitrile stabilizing solution to obtain glycine. The glycine nitrile stabilizing solution contains hydantoic acid amide and / or carbamyl acetonitrile, and the proportion of iminodiacetonitrile is not higher than 0.3%. The carbon source stabilizer is added in the glycolonitrile ammoniation process, so that the generation of iminodiacetonitrile in the ammoniation process can be reduced or even avoided, the main components of the obtained glycine nitrile stabilizing solution basically containing no iminodiacetonitrile are aminoacetonitrile, hydantoic acid amide and / or carbamyl acetonitrile, and the main components can be converted into glycine in the subsequent reaction; therefore, the generation of iminodiacetic acid and the accompanying separation problem are avoided.
Owner:LUFENG TIANBAO PHOSPHORUS CHEM CO LTD
Method for synthesizing alkyl pyrroles
InactiveCN105669514AHigh selectivityIncrease added valueCarboxylic acid nitrile preparationOrganic compound preparationDielectricPropanol
The invention aims to provide a method for synthesizing high-added-value alkyl pyrroles from methanol and ammonia by a one-step process. The method is characterized in that a non-equilibrium plasma-catalyst coupled mode is adopted to activate the raw materials methanol and ammonia so as to obtain the alkyl pyrroles. The method has the advantages of cheap and accessible reaction raw materials, mild reaction conditions, and simple and convenient operating technique. The conditions can be further regulated to synthesize methyl isonitrile, N,N-dimethyl cyanamide, aminoacetonitrile, N,N-dimethyl aminoacetonitrile and the like. The invention provides a novel method for preparing high-added-value chemicals by converting methanol. Besides, the method is suitable for synthesizing high-added-value nitrogenous compounds from one or two of methanol, ethanol and propanol and ammonia gas in the presence of the dielectric barrier plasma and catalyst.
Owner:DALIAN UNIV OF TECH
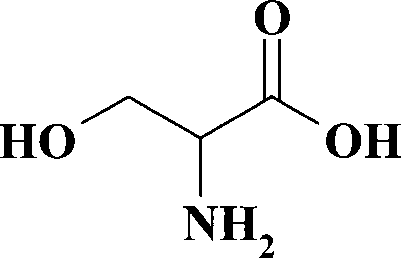
Method for preparing DL-serine
InactiveCN101168513AWide variety of sourcesHigh yieldOrganic compound preparationAmino-carboxyl compound preparationDecompositionSolvent
The invention discloses a preparation method of DL-serine. The invention adds methylamino methyl cyanide and paraformaldehyde into ethanol reaction solvent, and the addition reaction is performed with organic alkali triethylamine under the existence of catalytic agent, so as to obtain 1-methylol-1-methylamino methyl cyanide, and DL-serine of the invention is obtained through hydrochloric acid hydrolization; the yield rate is 86.7 to 88.9 percent, and the melting point is 245 DEG C (decomposition). Compared with the prior technology, the segregation operation of the invention is simple and convenient, the reactant raw material sources are extensive, the product yield rate is higher, the reproducibility is better, and the invention is suitable for the industrialized production.
Owner:SHANGHAI CHEM REAGENT RES INST
Method for synthesizing aminoacetonitrile hydrochloride from hydrocyanic acid
PendingCN111484426ASimple processMild reaction conditionsCarboxylic acid nitrile preparationOrganic compound preparationChemical synthesisSodium cyanide
The invention relates to the technical field of organic chemical synthesis. The method comprises the following steps: reacting hydrocyanic acid with formaldehyde to obtain a hydroxyacetonitrile aqueous solution, reacting hydroxyacetonitrile with ammonia to obtain an aminoacetonitrile aqueous solution, reacting aminoacetonitrile with hydrogen chloride, and concentrating, crystallizing, filtering and drying the obtained feed liquid to obtain aminoacetonitrile hydrochloride. The method provided by the invention is simple in technological process and mild in reaction condition, high-temperature and high-pressure operation is not involved, and used equipment is conventional equipment; except hydrocyanic acid, other raw materials are easy to obtain, and the raw material cost is low; waste waterand waste gas are small in amount and can be used indiscriminately, and only a small amount of ammonium chloride is generated; the product quality is good, and the appearance and content are higher than those of a sodium cyanide process.
Owner:营口德瑞化工有限公司
Method for preparing organic fertilizer by using aminonitrile waste liquid
InactiveCN101391911BSolve the emission problemAchieve reuseBio-organic fraction processingClimate change adaptationLiquid wasteOrganic manure
The invention provides a method for preparing an organic fertilizer by utilizing amino-nitrile waste liquor, which comprises the steps: catalyst and dispersant are added in the associated amino-nitrile waste liquor generated in the preparation of iminodiacetonitrile, aminoacetonitrile, ammonium dinitrile and EDTW, and mixed for reaction at the temperature of 30 to 150 DEG C and the pressure of 0 to 1.0MPa, so as to obtain base fertilizer which is added with biological strains for fermentation of 7 to 15 days at the temperature of 10 to 60 DEG C after nutrient allocation; and then hydrolysis is carried out to the base fertilizer to obtain a raw fertilizer which is carried out with nutrient allocation, pelleting and drying to be prepared into the organic fertilizer. The method can reduce the treatment cost, effectively utilizes the resources of the water liquor, and prepare the organic fertilizer by utilizing the amino-nitrile waste liquor.
Owner:四川省天然气化工研究院
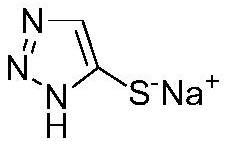
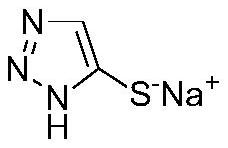
Synthesis method for preparing 5-sulfydryl-1, 2, 3-triazole sodium salt by one-pot method
ActiveCN112759556AImprove securityEase of industrial productionOrganic chemistryPtru catalystOrganic layer
The invention discloses a synthetic method for preparing 5-sulfydryl-1, 2, 3-triazole sodium salt by a one-pot method. The method comprises the following steps: (1) adding aminoacetonitrile hydrochloride into a solvent, and cooling; (2) dropwise adding the prepared oxidant into the solution in the step (1); (3) layering the solution in the step (2) to separate out an organic layer; (4) washing the organic phase in the step (3) with alkali liquor; (5) adding a catalyst, and dropwise adding the prepared sulfide solution into the solution in the step (4); (6) layering the solution in the step (5); and (7) adding the water phase in the step (6) into the prepared alkali liquor, carrying out a heating reaction, adjusting the pH value with acid after the reaction is finished, and separating out a solid, namely the 5-sulfydryl-1, 2, 3-triazole sodium salt product. The target compound is synthesized through the one-pot method, highly toxic hydrogen sulfide gas used in a traditional process is omitted, an intermediate with explosion danger is not separated, and safety is remarkably improved. The method is mild in reaction condition, simple in process and suitable for industrial production.
Owner:南京合创药业有限公司
A kind of synthetic method of 1-amino-1-cyclopropanenitrile hydrochloride
InactiveCN107652207BMild reaction conditionsHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationSulfonyl chloridePhenacyl
The invention discloses a synthesis method of 1-amino-1-cyclopropanecarbonitrile hydrochloride. The method includes: using aminoacetonitrile as the starting material to react with benzene sulfonyl chloride to obtain N, N-dibenzenesulfonyl acetonitrile, further carrying out ring closing reaction with 1, 2-dibromoethane under a sodium hydride alkaline condition to obtain N, N- benzenesulfonamidocyclopropanecarbonitrile, and finally conducting deprotection of benzoyl under an acidic condition so as to obtain 1-amino-1-cyclopropanecarbonitrile hydrochloride. The method provided by the invention has the advantages of mild reaction conditions, high yield and low cost, and is suitable for industrial application.
Owner:JIANGSU CANCER HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com