Synthesis method of alkylglycine
A technique for the synthesis of alkylglycine and a synthesis method, which is applied in the field of synthesis of alkylglycine, and can solve problems such as long reaction time, unsuitable synthesis method for 2-halogenated alkanes, difficulty in product separation, etc., and achieves convenient post-processing and is suitable for large-scale Produce, overcome expensive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
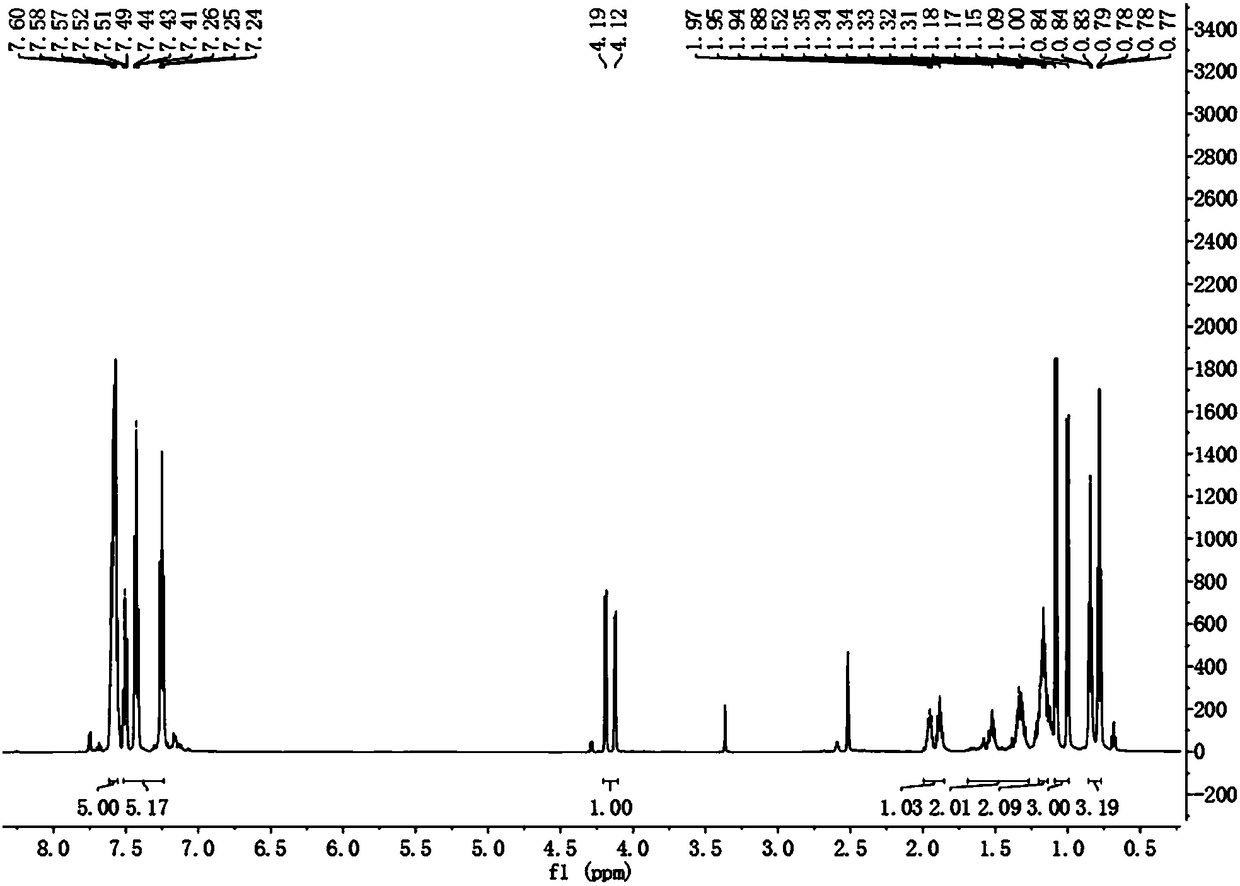
[0035] Embodiment 1: the synthetic method 1 of isopentylglycine
[0036] (1) Dissolve N-(dibenzylidene)aminoacetonitrile (I) (1.1g, 5mmol) in 30mL tetrahydrofuran, stir at -78°C (-70~-80°C) for about 10min, and then use Add lithium diisopropylamide (3.0mL, 6mmol, 1.2eq, within 7-8min) dropwise into the syringe, continue to stir for about 5min after the drop, then add 6mmol 2-halopentane (Ⅱ) dropwise with the syringe (can be Dissolve it in about 3-4 mL of tetrahydrofuran and add dropwise). After the dropwise addition is completed, turn off the refrigeration of the cryostat, stir and react for 1h (then check with TLC, if there are still a lot of raw materials that have not finished reacting, stir and react at room temperature for 1h, if the reaction is still not finished, take it out and heat it to reflux for a while Reaction 30min).
[0037] After the reaction was completed, the reaction mixture was poured into 100 mL of ethyl acetate and 20 mL of ammonium chloride solution (...
Embodiment 2
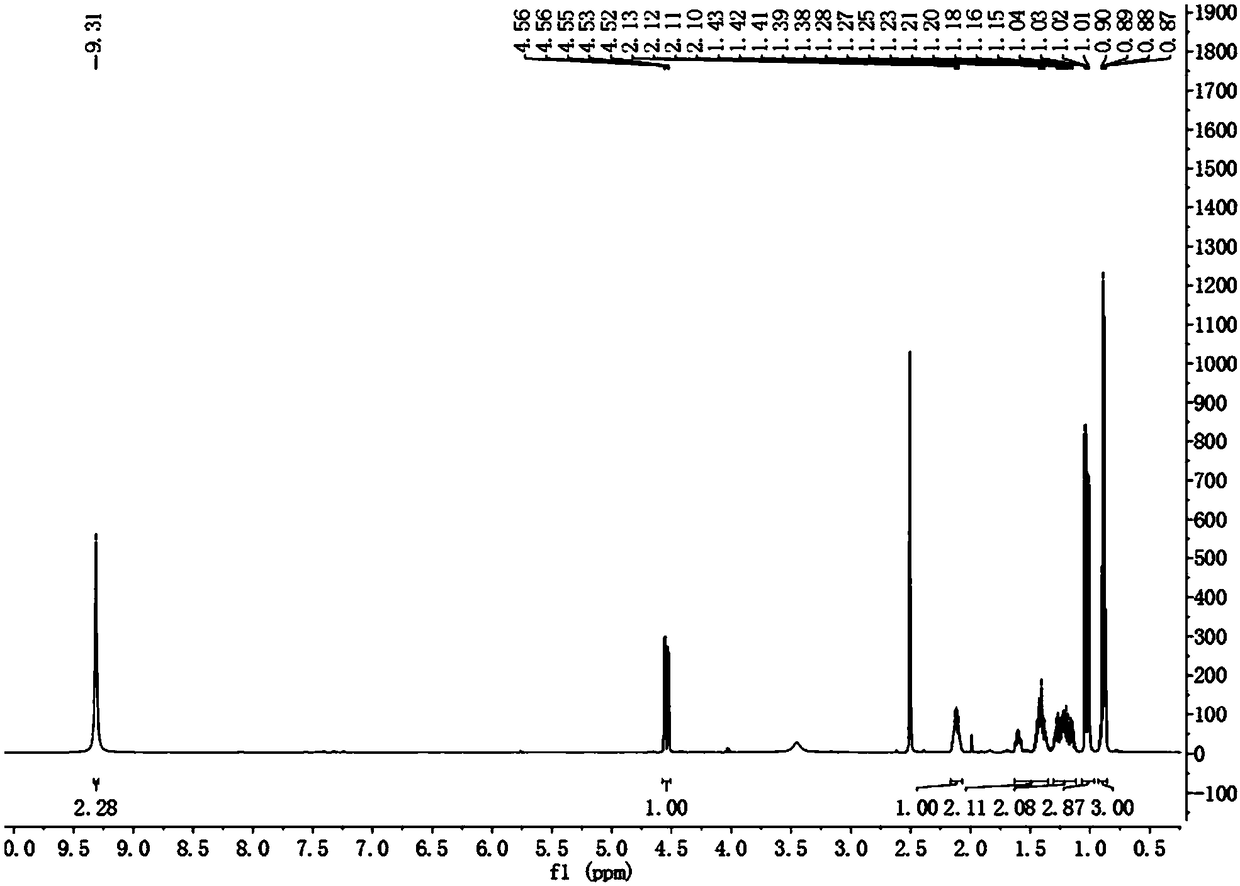
[0041] Embodiment 2: the synthetic method 2 of isopentylglycine
[0042] Dissolve N-(dibenzylidene)aminoacetonitrile (1.1g, 5mmol) in 30mL tetrahydrofuran, stir at -50°C (-40~-60°C) for about 10min, then add diisopropyl dropwise with a syringe Lithium amide (3.0mL, 6mmol, 1.2eq, drop within 7-8min), continue to stir for about 5min after dropping, and then add 6mmol 2-halopentane dropwise with a syringe (it can be dissolved to about 3-4mL tetrahydrofuran dropwise), other steps are the same as in Example 1. Under this temperature condition, the yield of the final product reached 74%.
Embodiment 3
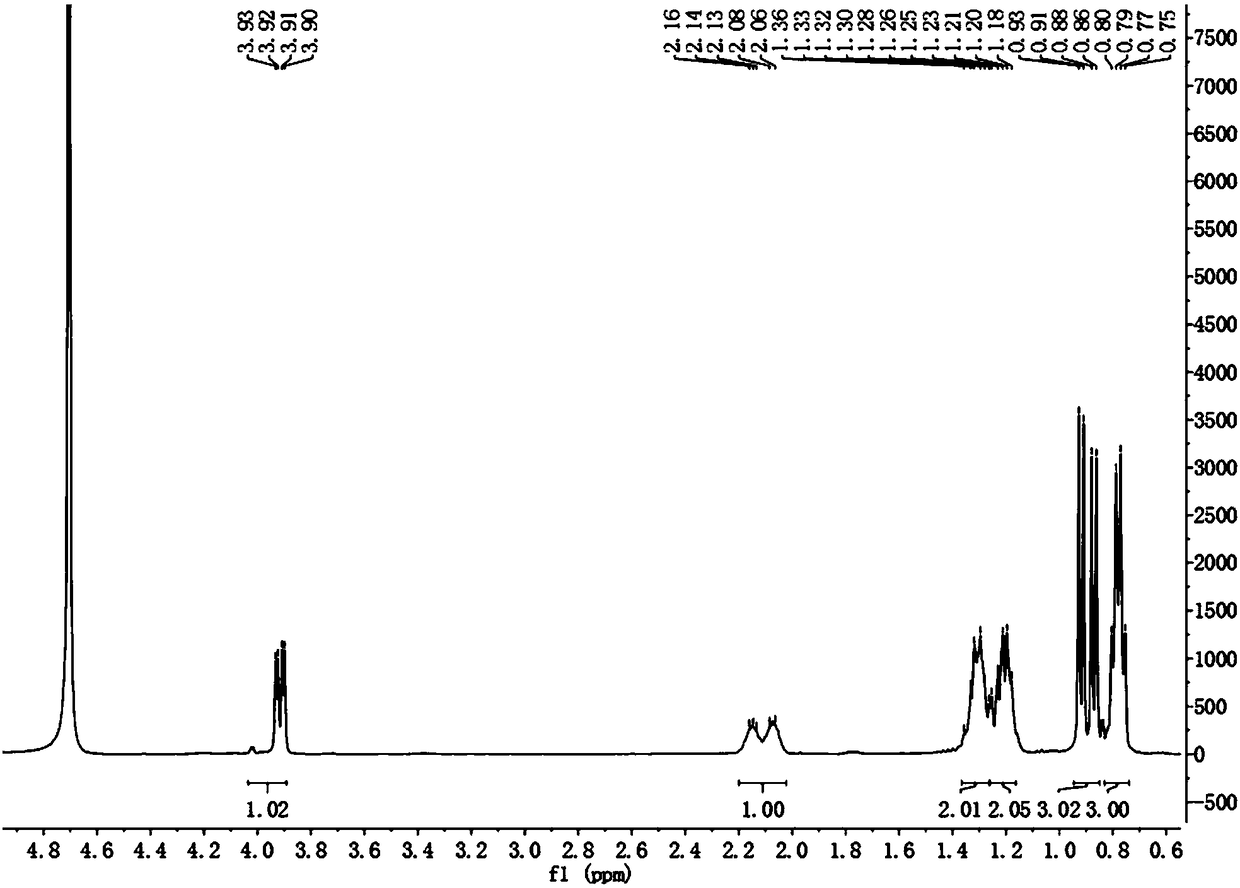
[0043] Embodiment 3: the synthetic method 3 of isopentylglycine
[0044] Dissolve N-(dibenzylidene)aminoacetonitrile (1.1g, 5mmol) in 30mL tetrahydrofuran, stir at -25°C (-20~-40°C) for about 10min, then add diisopropyl dropwise with a syringe Lithium amide (3.0mL, 6mmol, 1.2eq, drop in 7~8min), continue to stir for about 5min after dropping, and then add 6mmol 2-halopentane dropwise with a syringe (it can be dissolved to about 3~4mL tetrahydrofuran dropwise), other steps are the same as in Example 1. Under this temperature condition, the yield of the final product was 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com