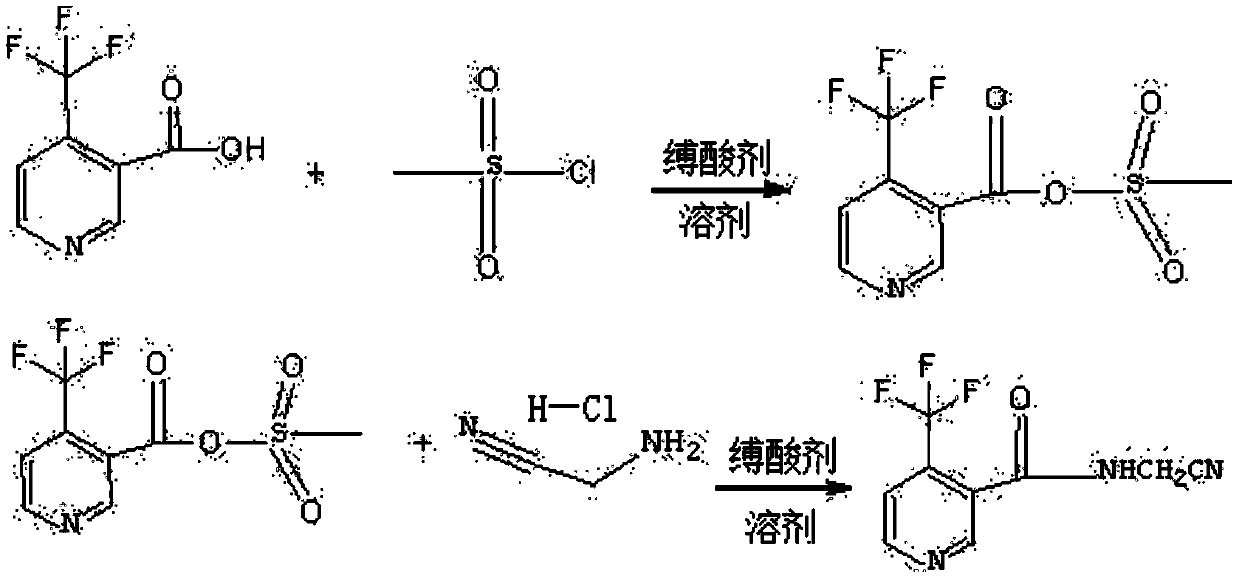
Synthesis method of N-cyanomethyl-4-(trifluoromethyl)-nicotinamide
A technology of trifluoromethyl nicotinic acid and trifluoromethyl, which is applied in the field of pesticides, can solve problems such as hydrolysis of acid chlorides, and achieve the effects of good yield, improved safety, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 15g (0.075mol) of 4-trifluoromethylnicotinic acid and 7.5g of toluene into a 250mL reactor with a reflux condenser, cool down to 5°C in ice water, and slowly add 2.88g (0.025mol) of methanesulfonyl chloride dropwise , No new products were detected by liquid phase detection, and the reaction temperature was raised to 20°C, and triethylamine was slowly added dropwise to the reaction system, and the reaction was stopped for 6 hours until no raw materials were tracked. Add 50 g of water to wash after the reaction, and dissolve the acid anhydride in the toluene layer for subsequent use.
[0030] In another 250ml reactor, add aminoacetonitrile hydrochloride 2.6g (0.0275mol), toluene 20g, cool to 0-5 ℃ in ice water, dropwise add 30% concentration of liquid caustic soda solution 7.45g, add 2.6 g sodium bicarbonate solid, then slowly drop the mixed acid anhydride obtained in the previous step into the system, react for 4 hours, and after liquid phase detection and tracking o...
Embodiment 2
[0032] Add 11g (0.055mol) of 4-trifluoromethylnicotinic acid and 2.2g of dichloroethane into a 250mL reactor with a reflux condenser, cool the ice water to 5°C, and slowly add 5.75g (0.050mol) Methanesulfonyl chloride, no new product was formed by liquid phase detection, and the reaction temperature was raised to 20°C, and triethylamine was slowly added dropwise to the reaction system, and the reaction was stopped for 8 hours until no raw materials were tracked. Add 50 g of water to wash after the reaction, and dissolve the acid anhydride in the dichloroethane layer for subsequent use.
[0033] In another 250ml reactor, add 4.3g (0.046mol) of aminoacetonitrile hydrochloride, 20g of dichloroethane, cool to 0-5°C in ice water, dropwise add 7.45g of 30% concentration of liquid caustic soda solution, and dropwise end Finally, add 3.45g of potassium carbonate solid, and then slowly drop the mixed acid anhydride obtained in the previous step into the system, react for 3 hours, liqui...
Embodiment 3
[0035] Add 5g (0.025mol) of 4-trifluoromethylnicotinic acid and 0.625g of N-methylpyrrolidone into a 250mL reactor with a reflux condenser, cool the ice water to 5°C, and slowly add 0.572g (0.005mol) ) methanesulfonyl chloride, no new product was generated by liquid phase detection, and the temperature of the system was raised to 40°C, and 3-picoline was slowly added dropwise to the reaction system, and the reaction was stopped for 6.5 hours until no raw materials were tracked. Add 50 g of water to wash after the reaction, and dissolve the acid anhydride in the toluene layer for subsequent use.
[0036] In another 250ml reactor, add aminoacetonitrile hydrochloride 1.576g (0.0166mol), N-methylpyrrolidone 20g, cool to 0-5 ℃ in ice water, dropwise add 7.45g of liquid caustic soda solution of 30% concentration, dropwise After the completion, add 0.66g of pyridine, then slowly drop the mixed acid anhydride obtained in the previous step into the system, react for 5.5h, liquid phase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com