Industrialized compounding method of N-alkyl substituted-5-cyanoimidazole compound
A cyanoimidazole and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems of long route, expensive raw materials, low yield, etc., and achieve the effect of low preparation cost and easy reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
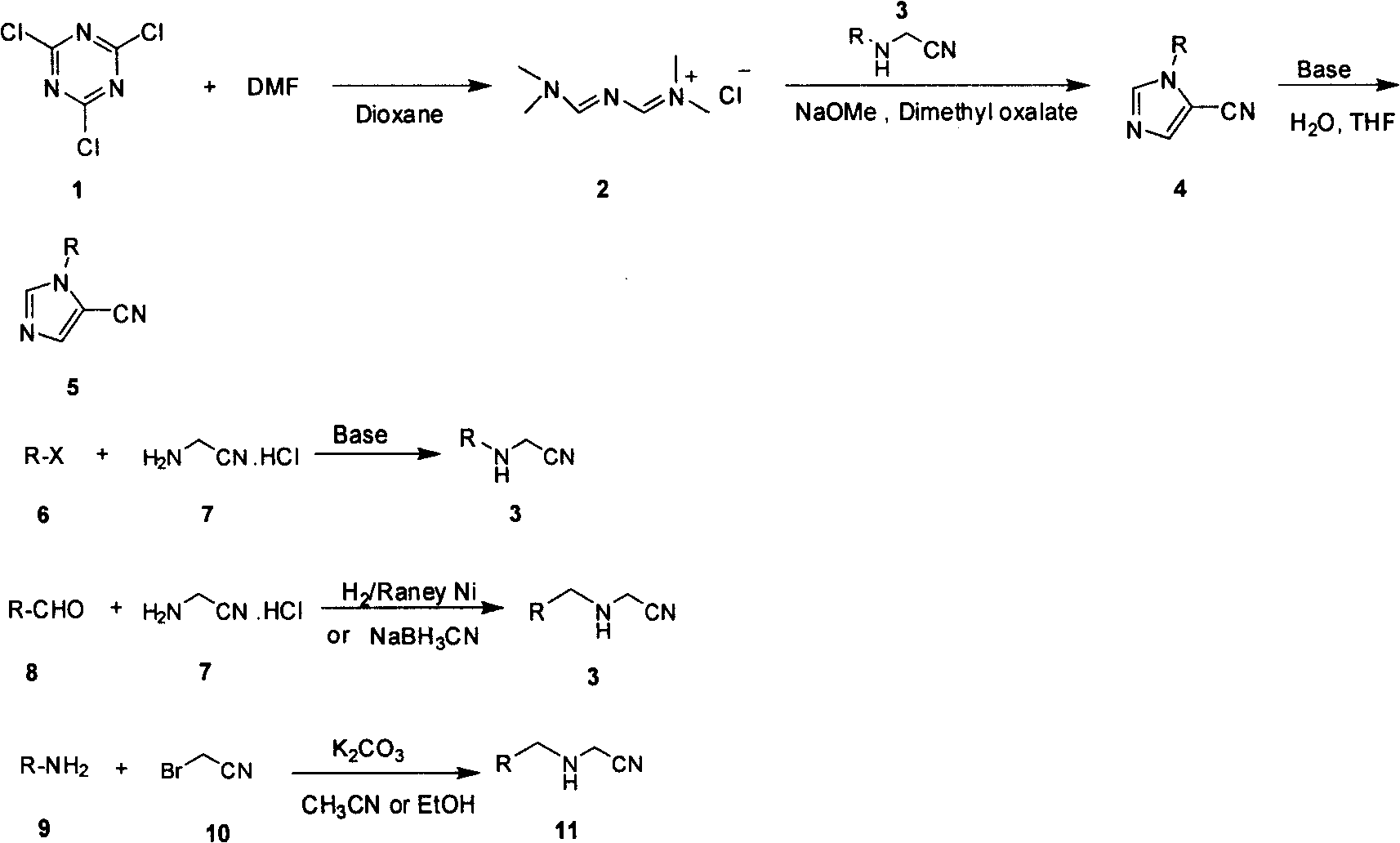
[0017] Synthesis of N-(((dimethylamino)methenylamino)methenyl)-N-dimethylammonium chloride (golden reagent)
[0018] Add cyanuric chloride (280g) and N,N-dimethylformamide (720g) to 1,4-dioxane (1500mL), heat to 65°C, react for one hour, then heat to 75-85°C, React for two hours. Cool to room temperature, filter, wash the filter cake with 1,4-dioxane (1000 mL), and dry under vacuum at low temperature to obtain 650 g of product, yield: 81%. 1 H NMR (400MHz, CDCl 3 ): δ9.58(s, 2H, CH, CH), 3.35(s, 6H, C 2 h 6 ), 3.17(s, 6H, CH 3 , CH 3 )
[0019] Synthesis of 1-methyl-5-cyanoimidazole
[0020] Acetonitrile hydrochloride (110 g) was added to tert-butyl methyl ether (2000 mL), followed by dimethyl oxalate (30 g) and freshly prepared 30% sodium methoxide in methanol (720 mL). Nitrogen was fed into the system, and gold reagent (240 g) was added simultaneously, allowing the reaction to take place in a nitrogen stream. Heated to 30°C for 24 hours. Cool to room temperatur...
Embodiment 2
[0022] Synthesis of Ethylaminoacetamide Hydrochloride
[0023] Ethylamine (18 g) was dissolved in ethanol (100 mL), and potassium carbonate (14 g) was added. Acetyl bromide (24 g) was slowly added dropwise under ice-bath conditions, and reacted at room temperature for 3 hours after the dropwise addition was complete. Filter, evaporate the organic solvent to dryness, add methyl tert-butyl ether solution (100 mL), let stand to separate the layers, and separate the lower aqueous phase. Add 5N hydrogen chloride in dioxane solution (30mL) to the organic matter in the upper layer, stir at room temperature for half an hour, filter, wash with dioxane (50mL) to obtain 17.5g product, yield: 63%. 1 H NMR (400MHz, DMSO-d 6 ): δ10.06(s, 2H, NH, HCl), 4.29(s, 2H, CH 2 ), 3.00 (m, 2H, CH 2 ), 1.21 (m, 3H, CH 3 ).
[0024] Synthesis of 1-ethyl-5-cyanoimidazole
[0025] Ethylaminoacetate hydrochloride (62.4 g) was added to tert-butyl methyl ether (1500 mL), followed by dimethyl oxal...
Embodiment 3
[0027] Synthesis of N-(((dimethylamino)methenylamino)methenyl)-N-dimethylammonium chloride (golden reagent)
[0028] Cyanuric chloride (28g) and N,N-dimethylformamide (72g) were added into tetrahydrofuran (150mL), heated to 65°C, reacted for one hour, then heated to reflux, reacted for two hours. Cool to room temperature, filter, wash the filter cake with tetrahydrofuran (100 mL), and dry under vacuum at low temperature to obtain 70 g of product, yield: 87%. Analysis data see embodiment 1.
[0029] Synthesis of 1-methyl-5-cyanoimidazole
[0030] Acetonitrile hydrochloride (10 g) was added to tetrahydrofuran (200 mL), followed by dimethyl oxalate (3 g) and potassium tert-butoxide (5 g). Nitrogen was passed into the system, and gold reagent (24 g) was added simultaneously, allowing the reaction to take place in a nitrogen stream. Heated to 40°C for 24 hours. After cooling to room temperature, the filtrate was evaporated to dryness and distilled under reduced pressure (80...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com