Glycine preparation method for reducing iminodiacetic acid content
A technology of iminodiacetic acid and glycine, which is applied in the preparation of carboxylic acid nitrile, the preparation of organic compounds, the preparation of cyanide reaction, etc., can solve the problems of high iminodiacetic acid content and difficult separation, and reduce the amount of urea added. The effect of avoiding separation problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 (add 0.2 equivalent of ammonium bicarbonate; in hydroxyacetonitrile, the same below)
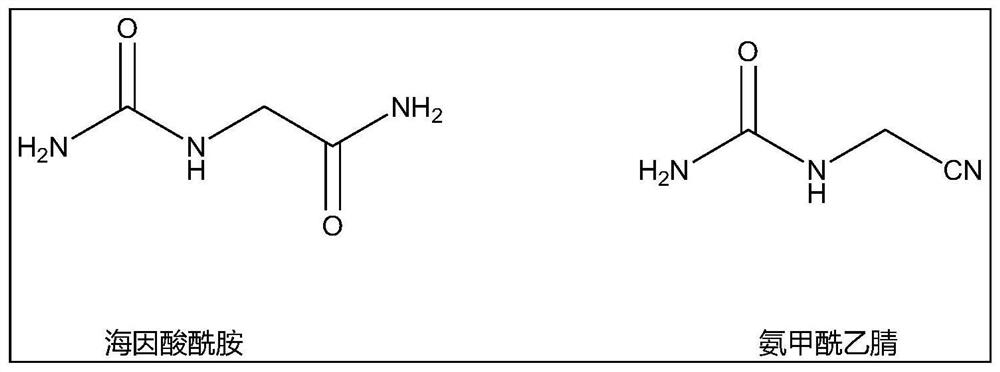
[0036] Mix 31.93g ammonium bicarbonate (99w%) carbon source stabilizer with 680g ammonia water (25w%) to obtain ammonia source liquid flow, measure 175.5g hydroxyacetonitrile (65w%, pH=4) as the hydroxyacetonitrile liquid flow, respectively pass through The material pump pumps the ammonia source liquid flow and the hydroxyacetonitrile liquid flow into the static mixer to mix and then send it to the tubular reactor. The ammoniation reaction is carried out at 115 ° C and the pressure of 1.3 MPa. The reaction residence time is 4 minutes. The glycine nitrile stabilized solution was obtained from the exit of the tubular reactor. HPLC analysis showed that no obvious iminodiacetonitrile was detected. In the product, aminoacetonitrile accounted for 82.6%, and hydantoin, carbamoylacetonitrile and other stable components accounted for 17.2%. %. The structural formulas of hydantoin an...
Embodiment 2
[0037] Example 2 (adding 0.2 equivalent of carbon dioxide)
[0038] Feed 17.6g carbon dioxide gas (0.4mol) carbon source stabilizer and mix with 680g ammonia water (25w%) to obtain ammonia source liquid flow, measure 175.5g hydroxyacetonitrile (65w%, pH=4) as the hydroxyacetonitrile liquid flow, respectively pass through The material pump pumps the ammonia source liquid flow and the hydroxyacetonitrile liquid flow into the static mixer to mix and then send it to the tubular reactor. The ammoniation reaction is carried out at 115 ° C and the pressure of 1.3 MPa. The reaction residence time is 3 minutes. Glycine nitrile stable solution was obtained from the exit of the tubular reactor. HPLC analysis showed that iminodiacetonitrile accounted for about 0.2%, aminoacetonitrile accounted for 86.4%, and hydantoin, carbamoylacetonitrile and other stable components accounted for 13.3%. %.
Embodiment 3
[0039] Example 3 (adding 0.15 equivalent of urea)
[0040] Mix 18.2g urea (99w%) carbon source stabilizer with 680g ammonia water (25w%) to obtain ammonia source liquid flow, measure 175.5g hydroxyacetonitrile (65w%, pH=2) as the hydroxyacetonitrile liquid flow, respectively pass through the material pump The ammonia source liquid flow and the hydroxyacetonitrile liquid flow are pumped into the static mixer and mixed, and then sent to the tubular reactor, and the ammoniation reaction is carried out at 130 ° C and the pressure of 1.8 MPa. The reaction residence time is 1 minute, and the reaction ends. Glycine nitrile stabilized liquid was obtained from the outlet of the reactor. HPLC analysis showed that no obvious iminodiacetonitrile was detected. The aminoacetonitrile accounted for 92.2% of the product, and hydantoin, carbamoylacetonitrile and other stable components accounted for 7.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com