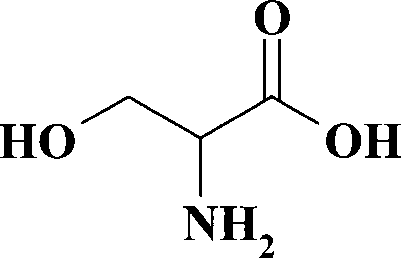
Method for preparing DL-serine
A serine and hydrochloric acid technology, applied in the chemical industry, can solve the problems of difficult separation and purification of the final product, obvious amplification effect, low reaction yield, etc., and achieve the effects of high product yield, easy separation and operation, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
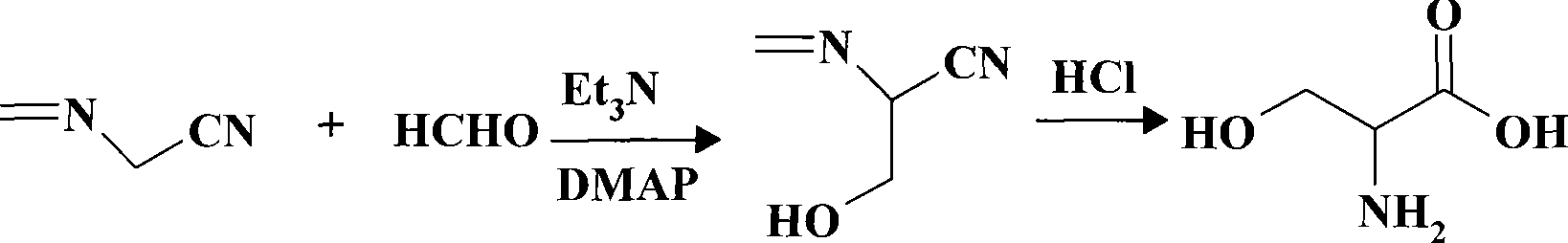
[0023] Add respectively 30ml absolute ethanol, 3.4g (0.05mol) methyleneamino acetonitrile, 1.8g (0.06mol) paraformaldehyde and 0.5g 4 -Dimethylaminopyridine (DMAP), raise the temperature under stirring and control the temperature at 55±5°C, slowly add 8.36ml (0.06mol) triethylamine dropwise, continue to react at 45±5°C for 4 hours after the drop, and use TLC Detection reaction end point (developing agent is V 石油醚 :V 乙酸乙酯 = 1: 1), after the reaction, adjust the pH of the solution with 5mol / l hydrochloric acid to be 7, then concentrate under reduced pressure to half of the original volume, reclaim 15ml of ethanol, leave it for 3 hours, and separate out 4.2g of white crystals, which is 1- Hydroxymethyl-1-methyleneaminoacetonitrile, m.p.64.0-64.5°C, yield 85.7%.
[0024] The above obtained 4.2g 1-hydroxymethyl-1-methyleneamino acetonitrile was added to 23ml of 10mol / l hydrochloric acid, heated under stirring, refluxed for 10 hours, and detected by TLC until the raw material poin...
Embodiment 2
[0026] Add 60ml absolute ethanol, 6.8g (0.10mol) methyleneamino acetonitrile, 3.6g (0.12mol) paraformaldehyde and 0.05g 4 - Dimethylaminopyridine (DMAP), raise the temperature under stirring and control the temperature at 55±5°C, slowly add 16.72ml (0.12mol) triethylamine dropwise, continue to react at 45±5°C for 5 hours after the drop, and use TLC Detection reaction end point (developing agent is V 石油醚 :V 乙酸乙酯 =1:1), after the reaction, adjust the pH of the solution to 7 with 5mol / l hydrochloric acid, then concentrate under reduced pressure to half of the original volume, reclaim 30ml of ethanol, leave it standing, and separate out 6.4g of white crystals, which is 1-hydroxymethyl Base-1-methyleneaminoacetonitrile, m.p.63-65°C, yield 65.3%.
[0027] The 6.4g 1-hydroxymethyl-1-methyleneamino acetonitrile obtained above was added in 23ml of 10mol / l hydrochloric acid, heated under stirring, refluxed for 12 hours, and detected by TLC until the raw material point disappeared (dev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com