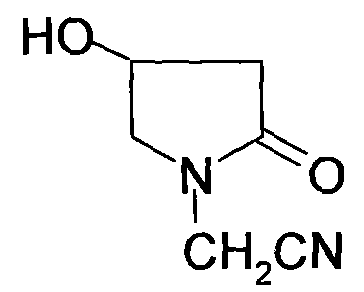
Method for preparing 4-hydroxylethylpyrrolidone-2-acetamide
A technology for hydroxypyrrolidone and acetamide, which is applied in the field of preparing 4-hydroxypyrrolidone-2-acetamide, can solve the problems that the purity cannot be fully used in medicine, cannot meet industrial scale production, and the product components are complicated, etc., and achieve cheap raw materials, The effect of low cost and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
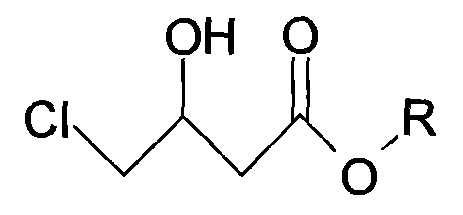
[0029] 1, preparation of
[0030] Add 500 ml of ethanol and 84.80 g (0.5 mol, purity 97%) of ethyl chloroacetoacetate into the reactor. The mixture was cooled to 3-8°C while stirring, and 6.75 g (0.125 mol) of potassium borohydride was added to react for 6 hours. Concentrate under reduced pressure to remove the solvent, and then rectify to obtain 75.30 g of ethyl chlorohydroxybutyrate, the content of gas chromatography analysis is 97.35%, and the yield is 88.05%.
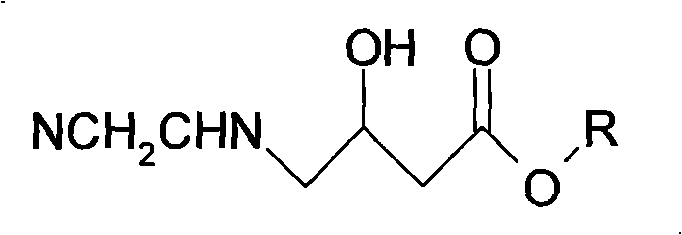
[0031] 2, preparation of
[0032] At room temperature, the product synthesized in the previous step was dissolved in 450ml of ethanol, and aminoacetonitrile hydrochloride 40.70g (0.44mol, purity 98.5%) and anhydrous sodium carbonate 46.65g (0.44 mol), after the addition, the temperature was raised to 65°C, and then heated to 80-85°C after 4 hours of heat preservation and reaction, and kept stirring and refluxed for 6 hours, and obtained by liquid chromatography analysis The solid residue in the reaction solu...
Embodiment 2
[0036] 1, preparation of
[0037] Add 500 ml of ethanol and 84.80 g (0.5 mol, purity 97%) of ethyl chloroacetoacetate into the reactor. The mixture was cooled to 3-8°C while stirring, and 6.75 g (0.125 mol) of potassium borohydride was added to react for 6 hours. Concentrate under reduced pressure to remove the solvent, and then rectify to obtain 74.30 g of ethyl chlorohydroxybutyrate, the content of gas chromatography analysis is 97.45%, and the yield is 86.97%.
[0038] 2, preparation of
[0039] At room temperature, the product obtained in the previous step synthesis was dissolved in 450ml of ethanol, and aminoacetonitrile sulfate 45.68g (0.435mol, purity 98.5%) and anhydrous sodium carbonate 46.10g (0.435mol) were added in batches to this mixed solution while stirring. ), heated up to 65°C after adding, heated to 80-85°C after 4 hours of heat preservation reaction, and kept stirring and refluxed for 6 hours, and obtained by liquid chromatography analysis After cool...
Embodiment 3
[0043] 1, preparation of
[0044] Add 500 ml of ethanol and 84.80 g (0.5 mol, purity 97%) of ethyl chloroacetoacetate into the reactor. The mixture was cooled to 10-15°C while stirring, and 6.75 g (0.125 mol) of potassium borohydride was added to react for 6 hours. Concentrate under reduced pressure to remove the solvent, and then rectify to obtain 70.80 g of ethyl chlorohydroxybutyrate, the content of gas chromatography analysis is 97.05%, and the yield is 82.53%.
[0045] 2, preparation of
[0046] At room temperature, the product synthesized in the previous step was dissolved in 450ml of ethanol, and aminoacetonitrile hydrochloride 38.20g (0.413mol, purity 98.5%) and anhydrous sodium carbonate 43.78g (0.413 mol) was added and the temperature was raised to 65°C, and then heated to 80-85°C after 4 hours of heat preservation and reaction, and kept stirring and refluxed for 6 hours, and obtained by liquid chromatography analysis After cooling to room temperature, the so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com