Method for preparing aminoacetonitrile and N,N-dimethylcyanamide from methane and ammonia gas through plasma synthesis
A technology for synthesizing aminoacetonitrile and dimethylcyanamide, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid nitriles, etc., can solve the problems of uninvolved and environmental pollution costs, and achieve the effect of simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
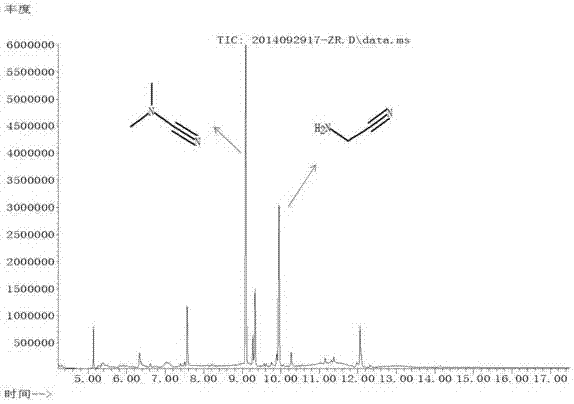
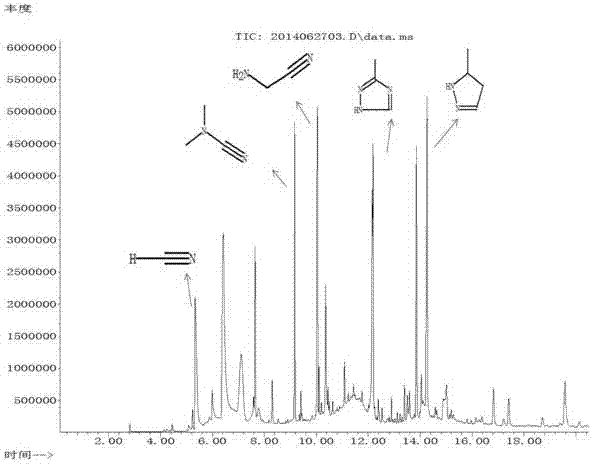
Image
Examples
Embodiment 1
[0059] Example 1: Single dielectric barrier discharge --- wire barrel reactor
[0060] Under the pressure of 0.1MPa, methane and ammonia are passed into the discharge reactor at a molar ratio of 1:2 (the flow rate of methane is 20ml / min, and the flow rate of ammonia is 40ml / min). After the gas flow is stable, the plasma power supply is turned on. Dielectric barrier discharge. The reactor adopts a wire cylinder electrode structure, and a cylindrical reactor is made of a hard glass tube with an outer diameter of 9mm and an inner diameter of 6mm (also used as a barrier medium). The central electrode is a stainless steel electrode with a diameter of 3mm, and the ground electrode has a cylindrical wall thickness of 1mm. Shaped iron foil (close to the outer wall of the glass tube), the pole spacing is 3mm, and the effective discharge length of the reactor is 100mm.
[0061] The discharge parameters of the reactor are: voltage 12kV, frequency 12kHz; other reaction conditions of the ...
Embodiment 2
[0063] Repeat Example 1, but change the pressure to 0.05MPa, and keep the other parameters unchanged. The result of the reaction is: the methane conversion rate is 10%, there is carbon deposition, the selectivity of aminoacetonitrile is 10%, and the selectivity of N,N-dimethylcyanamide is 15%.
Embodiment 3
[0065] Repeat Example 1, but change the molar ratio of methane and ammonia to 1:3 (wherein the methane flow rate is 15ml / min, the ammonia flow rate is 45ml / min), and the remaining parameters are unchanged. The reaction results are: the methane conversion rate is 7%, the aminoacetonitrile selectivity is 35%, and the N,N-dimethylcyanamide selectivity is 43%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com