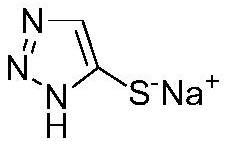
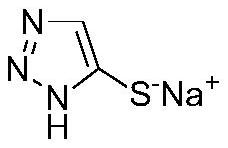
Synthesis method for preparing 5-sulfydryl-1, 2, 3-triazole sodium salt by one-pot method
A synthesis method and technology of sodium triazole are applied in the field of one-pot preparation of 5-mercapto-1, and can solve the problems of short steps, dangerous separation process and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In the reaction flask, add 50ml of water, 31g of aminoacetonitrile hydrochloride, stir, dissolve, add 180ml of dichloromethane, cool to -5°C-0°C, dropwise add 80g of sodium nitrite solution (37.5%), and control the dropwise Adding temperature 1 HNMR (D 2 O, 400MHz): δ7.20(s, 1H, PhH); MS, m / z: 123.99[M+H] +
Embodiment 2
[0039] In the reaction bottle, add 50ml of water, 31g of aminoacetonitrile hydrochloride 31g, stir, dissolve, add 83ml of dichloromethane, 98ml of chloroform, cool to -5°C-0°C, dropwise add 80g of sodium nitrite solution ( 37.5%), control the dropwise addition temperature1 HNMR (D 2 O, 400MHz): δ7.20(s, 1H, PhH); MS, m / z: 123.99[M+H] +
Embodiment 3
[0041]In the reaction flask, add 50ml of water, 31g of aminoacetonitrile hydrochloride, stir, dissolve, add 83ml of dichloromethane, 98ml of chloroform, cool to -5°C-0°C, dropwise add 80g of sodium nitrite solution (37.55 ), control the dropwise addition temperature1 HNMR (D 2 O, 400MHz): δ7.20(s, 1H, PhH); MS, m / z: 123.99[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com