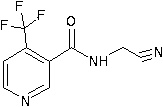
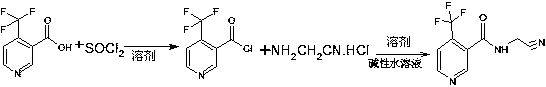N-cyanomethyl-4-(trifluoromethyl) nicotinamide preparation method
A technology of trifluoromethyl and cyanomethyl, applied in the field of preparation of N-cyanomethyl-4-(trifluoromethyl)nicotinamide, can solve the problems of high production cost, difficult industrialization, low yield and the like, Achieve the effect of low production cost, easy operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Put 30.0 g (0.157 mol) of 4-(trifluoromethyl) nicotinic acid and 100 ml of benzene into a 250 ml four-neck flask equipped with a reflux condenser, a thermometer, and a dropping funnel, heat up to 50-60 ° C, drop Add 28.0 g (0.235 mol) of thionyl chloride for about 1 hour. After the dropwise addition, the temperature is raised to 80° C. and kept for 2 hours. After the reaction is completed, 4-(trifluoromethyl)nicotinoyl chloride benzene solution is obtained for later use.
[0025] In another 1000 ml four-necked flask with a thermometer and a dropping funnel, 33.3 grams (0.314 mol) of sodium carbonate and 133.2 grams of tap water were put in, and after stirring for 10 minutes, 120 ml of benzene and 21.79 grams of aminoacetonitrile hydrochloride (0.235 mol), then the temperature was lowered to 10°C under stirring, and the 4-(trifluoromethyl)nicotinoyl chloride benzene solution was added dropwise, and the addition was completed in about 3 hours, and the temperature was kept ...
Embodiment 2
[0027] Put 30.0 g (0.157 mol) of 4-(trifluoromethyl) nicotinic acid and 100 ml of toluene into a 250 ml four-necked flask equipped with a reflux condenser, a thermometer, and a dropping funnel, heat up to 50-60 ° C, drop Add 28.0 g (0.235 mol) of thionyl chloride for about 1 hour. After the dropwise addition, the temperature is raised to 80° C. and kept for 2 hours. After the reaction is completed, 4-(trifluoromethyl)nicotinoyl chloride benzene solution is obtained for later use.
[0028] In another 1000 ml four-necked flask with a thermometer and a dropping funnel, 33.3 grams (0.314 mol) of sodium carbonate and 133.2 grams of tap water were put in, and after stirring for 10 minutes, 120 milliliters of toluene and 21.79 grams of aminoacetonitrile hydrochloride (0.235 mol), then the temperature was lowered to 10°C under stirring, and the 4-(trifluoromethyl)nicotinoyl chloride benzene solution was added dropwise, and the addition was completed in about 3 hours, and the temperatur...
Embodiment 3
[0030] 30.0 g (0.157 mol) of 4-(trifluoromethyl) nicotinic acid and 100 ml of dichloroethane were put into a 250 ml four-neck flask with a reflux condenser, a thermometer, and a dropping funnel, and the temperature was raised to 50-60 ℃, add 28.0 g (0.235 mol) of thionyl chloride dropwise, and finish adding in about 1 hour. After the dropwise addition is completed, raise the temperature to 80°C and keep it for 2 hours. After the reaction is completed, 4-(trifluoromethyl)nicotinoyl chloride benzene solution is obtained .
[0031] In another 1000 ml four-necked flask with a thermometer and a dropping funnel, put 33.3 grams (0.314 mol) of sodium carbonate and 133.2 grams of tap water into it. After stirring for 10 minutes, put 120 milliliters of dichloroethane and 21.79 grams of aminoacetonitrile hydrochloride. gram (0.235mol), then lower the temperature to 10°C while stirring, and start to drop the 4-(trifluoromethyl)nicotinoyl chloride benzene solution for use in the previous s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com