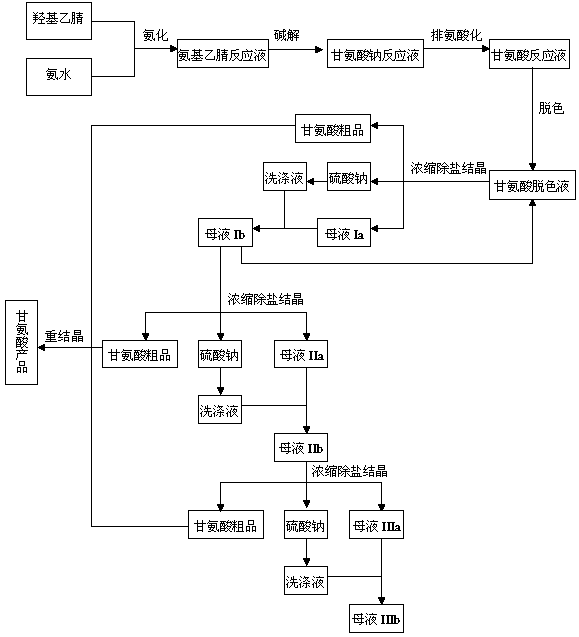
Preparation process of glycine
A preparation process, glycine technology, applied in the chemical industry, can solve the problems of prolonged concentration time, large volume of alkaline solution, and high production cost, and achieve the effects of reducing raw material decomposition, shortening reaction time, and improving production capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The first batch of production specifically includes the following steps:
[0028] a. Ammonification: Mix 228.06 g of hydroxyacetonitrile and 1175 g of ammonia water with a concentration of 23% by mass (the molar ratio of hydroxyacetonitrile to ammonia is 1:4), and pump it into a tubular reactor at a temperature of 83°C and a pressure of Under the condition of 1.3MPa, the ammoniation reaction was carried out, and the reaction time was adjusted to be 6 minutes by controlling the flow rate to obtain the aminoacetonitrile reaction solution;
[0029] b. Alkaline hydrolysis: Add 351.82 g of sodium hydroxide solution with a mass percent concentration of 50% in advance in the alkaline hydrolysis reactor (the molar ratio of sodium hydroxide to hydroxyacetonitrile is 1.1:1), and use the alkaline hydrolysis reactor in the tubular reaction The aminoacetonitrile reaction liquid is collected at the outlet of the reactor, and the aminoacetonitrile reaction liquid that enters the alk...
Embodiment 2
[0040] The first batch of production specifically includes the following steps:
[0041] a. Ammonification: Mix 228.06g of hydroxyacetonitrile and 881.3g of ammonia water with a concentration of 23% by mass (the molar ratio of hydroxyacetonitrile to ammonia is 1:3), pump it into the tubular reactor, and heat it at a temperature of 50°C and a pressure of Under the condition of 2.0MPa, the ammoniation reaction was carried out, and the reaction time was adjusted to be 4 minutes by controlling the flow rate to obtain the aminoacetonitrile reaction solution;
[0042] b. Alkaline hydrolysis: add 416g of sodium hydroxide solution with a concentration of 50% by mass in advance in the alkaline hydrolysis reactor (the molar ratio of sodium hydroxide to hydroxyacetonitrile is 1.3:1), and use the alkaline hydrolysis reactor in the tubular reactor The outlet of the aminoacetonitrile reaction liquid is collected, and the aminoacetonitrile reaction liquid entering the alkali hydrolysis rea...
Embodiment 3
[0053] The first batch of production specifically includes the following steps:
[0054] a. Ammonification: Mix 228.06 g of hydroxyacetonitrile and 1762.5 g of ammonia water with a concentration of 23% by mass (the molar ratio of hydroxyacetonitrile to ammonia is 1:6), and pump them into the tubular reactor. Under the condition of 0.5MPa, the ammoniation reaction was carried out, and the reaction time was adjusted to be 10 minutes by controlling the flow rate to obtain the aminoacetonitrile reaction solution;
[0055] b. Alkaline hydrolysis: Add 384g of sodium hydroxide solution with a mass percentage concentration of 50% in advance in the alkaline hydrolysis reactor (the molar ratio of sodium hydroxide to hydroxyacetonitrile is 1.2:1), and use the alkaline hydrolysis reactor in the tubular reactor The aminoacetonitrile reaction solution is collected at the outlet of the outlet, and the aminoacetonitrile reaction solution entering the alkaline hydrolysis reactor immediately ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com