Method for synthesizing methionine hydroxy analogue by continuous and rapid hydrolysis of 2-hydroxy-4-methylthio butyronitrile
A technology of methylthiobutyronitrile and methionine hydroxyl, which is applied in the chemical industry, can solve the problems of incomplete hydration and hydrolysis, dark color of methionine hydroxyl analogs, and long reaction time, so as to reduce the polymerization of raw materials and the formation of by-products, Increase the collision between molecules, the effect of high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
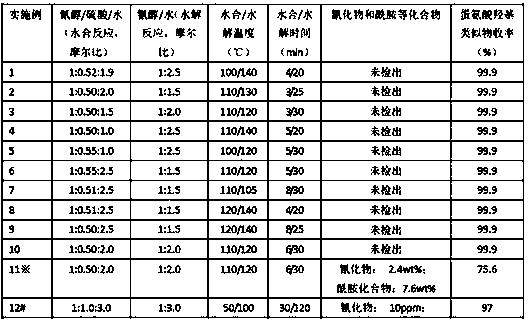
[0029] 163.995 grams (1.0 mol) of 2-hydroxy-4-methylthiobutyronitrile (cyanohydrin) aqueous solution with a mass percentage of 80 wt% and 52.0 grams (0.52 moles) of sulfuric acid with a mass percentage of 98 wt% were simultaneously measured The pump pumps the loaded solid catalyst (SO 4 2- / Fe 2 o 3), the molar ratio of 2-hydroxy-4-methylthiobutyronitrile (cyanohydrin), sulfuric acid and water is 1:0.52:1.90, and the aqueous solution of 2-hydroxy-4-methylthiobutyronitrile is in The flow rate in the microchannel is 9.0 g / min, the flow rate of sulfuric acid in the microchannel is 2.854 g / min, the controlled reaction temperature is 100°C, the reaction pressure is 0.2MPa, and the residence time is 4min (that is, the hydration reaction time is also the reaction time). The time when the liquid flows through the microchannel), the liquid flowing out from the microchannel is 2-hydroxy-4-methylthiobutyramide sulfate solution. 2-Hydroxy-4-methylthiobutanamide sulfate solution and 45...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com