A kind of method for continuous and rapid synthesis of 5-(β-methylthioethyl)-hydantoin
A technology of methylthioethyl and hydantoin, applied in organic chemistry, products, reagents, etc., can solve the problems of lack of economical efficiency, easy polymerization of raw material cyanohydrin, easy generation of by-products, etc. The effect of depolymerization and its by-product formation, shortening reaction time, and increasing intermolecular collisions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
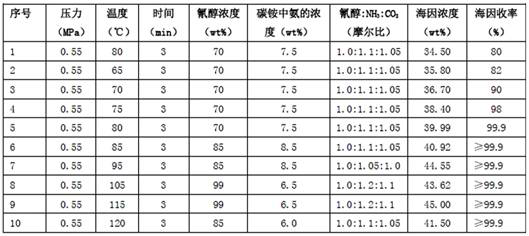
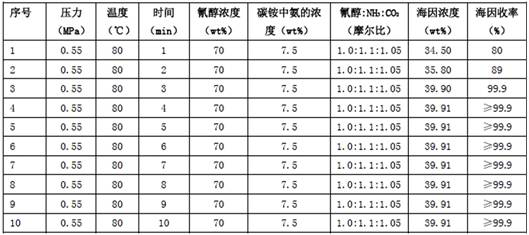
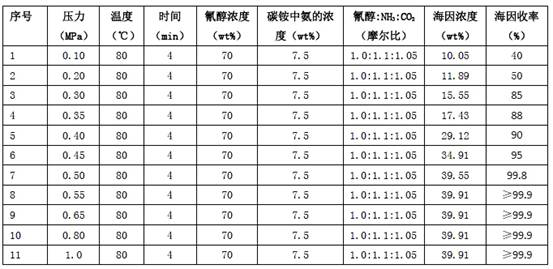
[0027] Example 1 Two-component reaction of cyanohydrin-ammonium bicarbonate aqueous solution
[0028] 93.711 g (0.5 mol) of 2-hydroxy-4-methylthiobutyronitrile aqueous solution (cyanohydrin) with a mass percentage content of 70 wt % and ammonium bicarbonate aqueous solution with ammonia and carbon dioxide contents of 7.5 wt % and 18.53 wt % respectively 124.667 g (cyanohydrin:ammonia:carbon dioxide=1.0:1.10:1.05) was pumped into the microchannel reactor loaded with catalyst N10 through a metering pump at the same time, the flow rate of cyanohydrin in the microchannel was 2.5ml / min, and the ammonium bicarbonate aqueous solution was The flow rate in the microchannel is 3.33ml / min, the temperature of the control reaction is 80°C, the pressure is 0.55MPa, the residence time is 3min (that is, the time for the reaction liquid to flow through the microchannel), and the outflow liquid is clear, colorless and transparent 5 -(β-Methylthioethyl)-hydantoin aqueous solution (hydantoin) 218...
Embodiment 2
[0046] Example 2 Three-component reaction of methylthiopropionaldehyde, sodium cyanide, and ammonium bicarbonate aqueous solution
[0047] 52.3473 g (0.5 mol) of methylthiopropionaldehyde with a mass percentage of 99.5 wt %, 82.4833 g (0.505 mol) of an aqueous sodium cyanide solution with a mass percentage of 30 wt %, and ammonia and carbon dioxide with a content of 7.5 wt % respectively. and 124.667 g of 18.53 wt% ammonium bicarbonate aqueous solution (methylthiopropionaldehyde: sodium cyanide: ammonia: carbon dioxide = 1.0: 1.01: 1.10: 1.05) simultaneously through a metering pump into the microchannel reactor loaded with catalyst N10, The flow rate of methylthiopropionaldehyde in the microchannel is 2.5ml / min, the flow rate of the aqueous sodium cyanide solution in the microchannel is 3.9393ml / min, and the flow rate of the aqueous ammonium bicarbonate solution in the microchannel is 5.954ml / min, controlling the reaction The temperature is 100°C, the pressure is 1.10MPa, the ...
Embodiment 3
[0048] Example 3 Three-component reaction of methylthiopropionaldehyde, potassium cyanide and ammonium bicarbonate aqueous solution
[0049] 52.3473 g (0.5 mol) of methylthiopropionaldehyde with a mass percentage of 99.5 wt %, 93.9531 g (0.505 mol) of an aqueous potassium cyanide solution with a mass percentage of 35 wt %, and ammonia and carbon dioxide with a content of 7.5 wt % respectively. and 124.667 g of 18.53 wt% ammonium bicarbonate aqueous solution (methylthiopropionaldehyde: potassium cyanide: ammonia: carbon dioxide = 1.0: 1.01: 1.10: 1.05) simultaneously through a metering pump into the microchannel reactor loaded with catalyst N10, The flow rate of methylthiopropionaldehyde in the microchannel is 2.5ml / min, the flow rate of the potassium cyanide aqueous solution in the microchannel is 4.487ml / min, and the flow rate of the aqueous ammonium bicarbonate solution in the microchannel is 5.954ml / min, controlling the reaction The temperature is 90°C, the pressure is 1.0M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com