Percutaneous absorption agent containing Ailamode, preparation method and medical uses thereof
A technology of transdermal absorbent and transdermal accelerator, which is applied in the field of transdermal absorbents containing iguratimod, which can solve problems such as difficulty in taking medicine, no report of topical transdermal absorption of iguratimod, and damage to the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
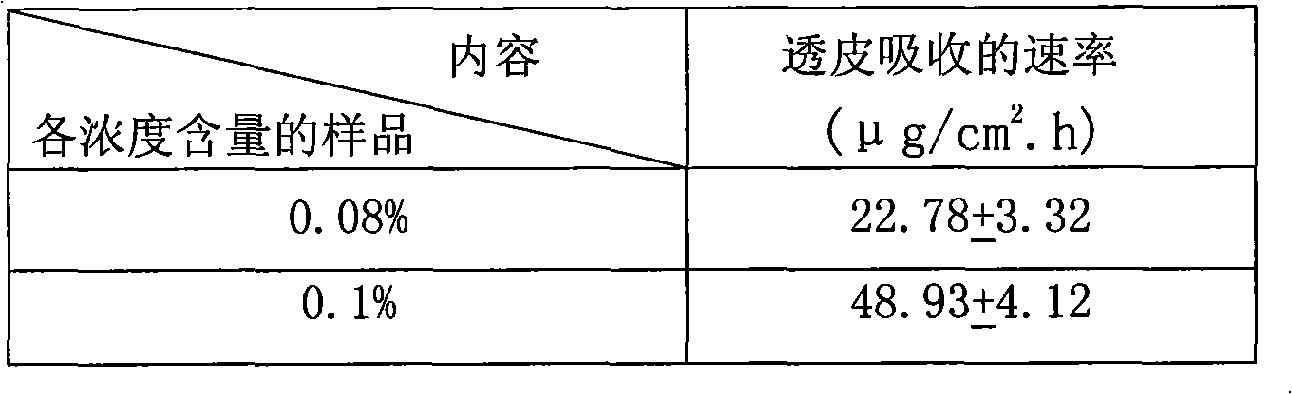
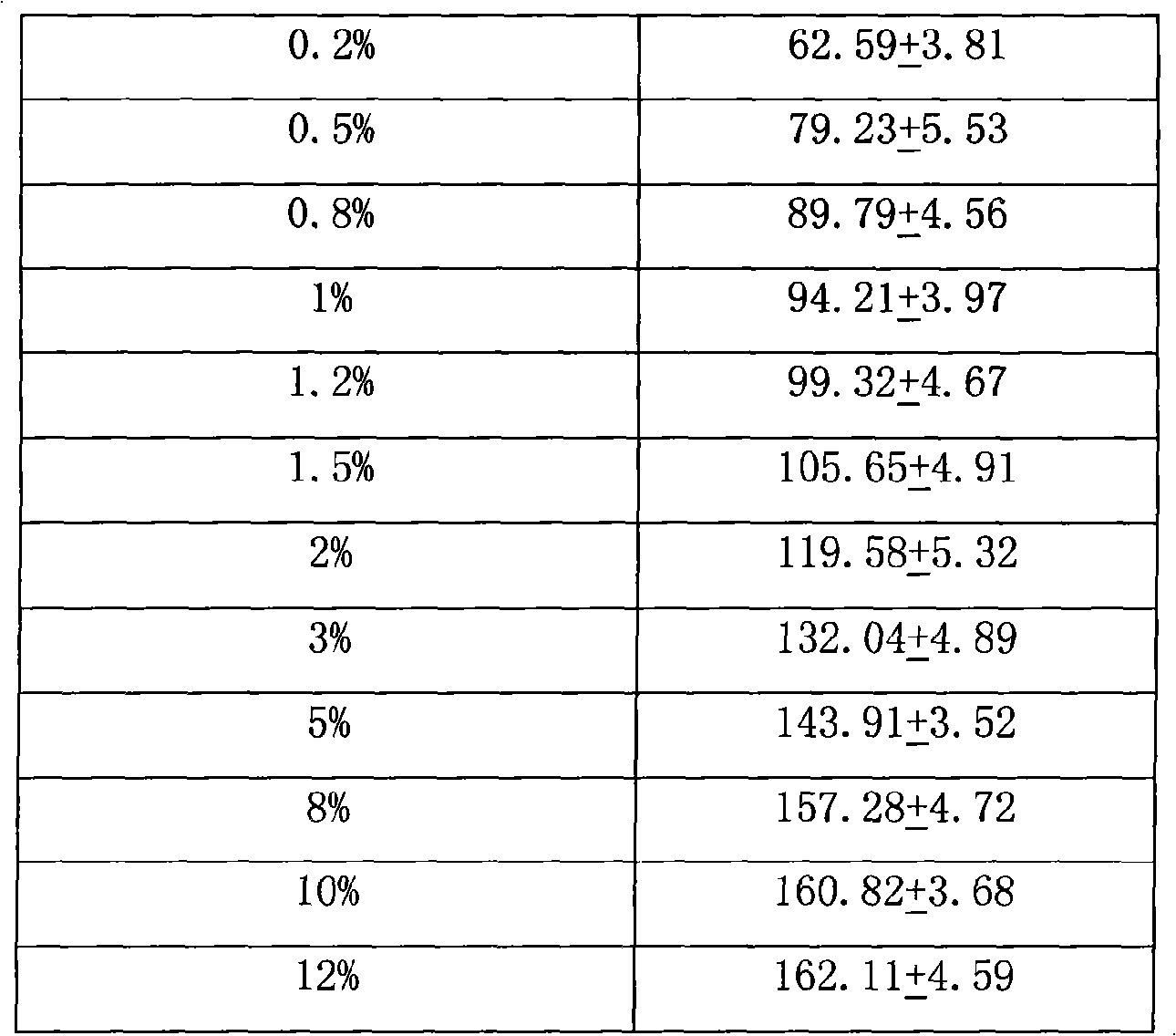
Image
Examples
Embodiment 1
[0157] Embodiment 1. Iguratimod gel and preparation thereof
[0158] Prescription: Iguratimod 0.5g, Carbomer 1g, Triethanolamine 2g, Propylene Glycol 10g, Laurocaprazine 2g, Glycerin 5g, Ethanol 5ml, Purified water to 100g.
[0159] Preparation: Take carbomer and add 35ml of purified water to make it fully swell, stir evenly to obtain a carbomer solution, adjust the pH value of the solution to 6.0-9.5 with triethanolamine, and stir well to obtain a blank gel; Dissolve Derong powder in ethanol, stir well, add propylene glycol, laurocapram, glycerin, stir evenly, then slowly add the liquid medicine into the carbomer matrix, stir evenly while adding, and finally add purified water to 100g, Get the iguratimod gel, packed in an aluminum tube, every 20g, to get final product.
Embodiment 2
[0160] Embodiment 2. Iguratimod gel and its preparation
[0161] Prescription: Iguratimod 1g, chitosan 1.5g, triethanolamine 1g, glycerin 8g, laurocapram 2g, ethanol 35ml, distilled water to 100g.
[0162] Preparation: Take iguratimod and glycerin, add 35ml of ethanol, stir evenly, add 50ml of distilled water to dissolve and dilute, sprinkle chitosan on the surface of the liquid, make it fully swell, stir well, add laurocaprolactone, stir well, and then Add triethanolamine, stir well, add distilled water to 100g, stir evenly to obtain Airamod gel, pack it.
Embodiment 3
[0163] Embodiment 3. Iguratimod gel and its preparation
[0164] Prescription: Ilamod 2g, Carbomer 9403g, Menthol 2.5g, Propylene Glycol 20g, Isopropanol 12g, Ethylparaben 0.2g, Triethanolamine amount, Purified water up to 100g.
[0165] Preparation: Sprinkle Carbomer 940 evenly in an appropriate amount of purified water to make it fully swell, add purified water to 50ml, and stir evenly to obtain a Carbomer solution. Use triethanolamine to adjust the pH value of the solution to 6.0-8, and stir well. Obtain a blank gel; add iguratimod, menthol, ethyl paraben, add isopropanol and propylene glycol to dissolve, add slowly while stirring the blank gel, stir evenly, add water to 100g, stir well, and get Aguratimod gel, ready to pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com