Medicinal composition capable of preventing or treating inflammatory diseases
A composition and drug technology, applied in the direction of drug combination, allergic diseases, antipyretics, etc., can solve the problems of many adverse reactions and restrictions on widespread use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
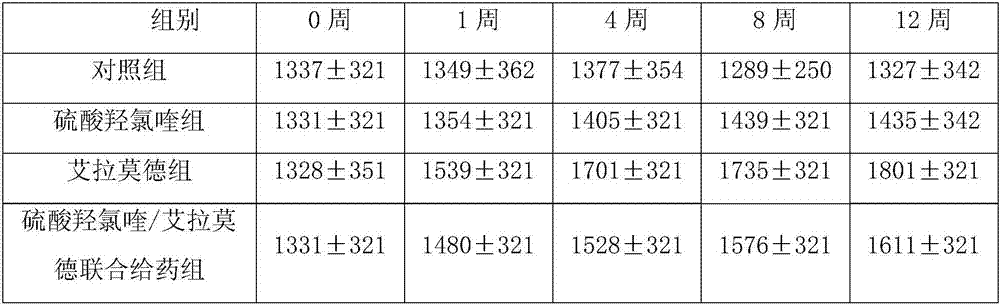
[0025] Embodiment 1: The effect of hydroxychloroquine sulfate and iguratimod composition in treating arthritis in mice
[0026]Dosage group design: normal control group, model control group (normal saline), hydroxychloroquine sulfate (15mg / kg) group, iguratimod (1mg / kg) group, iguratimod (2mg / kg) group, hydroxychloroquine sulfate Chloroquine / iguratimod combined administration 2.8:1 group (5mg / kg hydroxychloroquine sulfate + 2mg / kg iguratimod), hydroxychloroquine sulfate / iguratimod combined administration 8.4:1 group (15mg / kg sulfuric acid Hydroxychloroquine + 2mg / kg iguratimod), hydroxychloroquine sulfate / iguratimod combined administration 16.7:1 group (15mg / kg hydroxychloroquine sulfate + 1mg / kg iguratimod), the ratio is molar Than calculate.
[0027] Construction of collagen mouse arthritis animal model: mice were used as test animals, 80 SPF grade DBA / 1 mice, male, 7-8 weeks old, weighing 18-22g, were randomly divided into 8 groups. Except for the normal group, the mou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com