Iguratimod osmotic pump controlled slow-release preparation
A technology of controlled release and osmotic pump, applied in the field of medicine, to achieve stable blood drug concentration, lasting and constant drug release rate, and good correlation between in vitro and in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
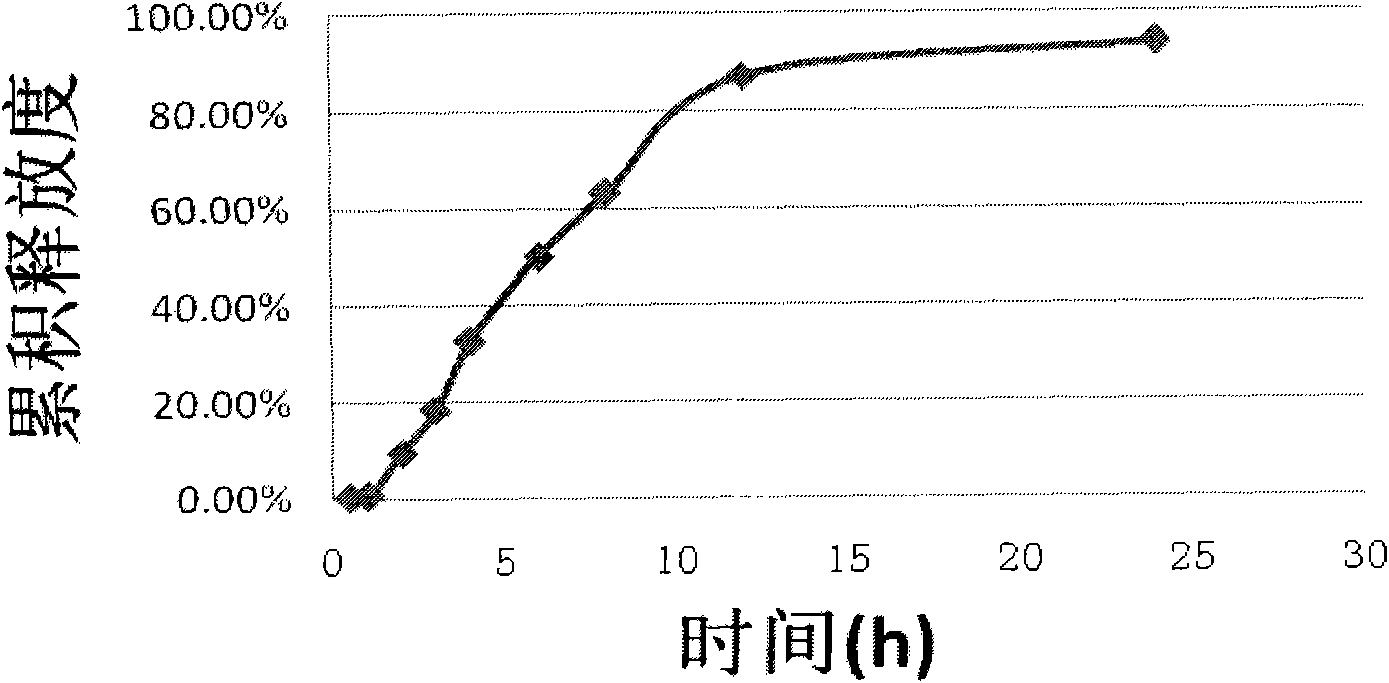
Embodiment 1
[0034] prescription:
[0035] Drug-containing layer (calculated by weight percentage of drug-containing layer)
[0036] Alamode 31.25%
[0037] Polyoxyethylene 62.5%
[0038] appropriate amount of lemon yellow
[0039] Sodium Lauryl Sulfate 4.38%
[0041] 5% PVA ethanol solution appropriate amount
[0042] Booster layer (calculated by weight percentage of booster layer)
[0043] Polyoxyethylene 62.5%
[0045] Hydroxypropyl Methyl Cellulose 3.12%
[0046] Polyvinylpyrrolidone k30 6.25%
[0047] Iron oxide red amount
[0049] 8% PVA ethanol solution appropriate amount
[0050] Controlled release film (by weight percentage of tablet core)
[0051] Cellulose acetate 7.92%
[0052] Polyethylene glycol 4000 0.264%
[0053] Acetone: water appropriate amount
[0054] Protective layer: (by weight percentage of coated tablet)
[0055] Opadry Gastric Coating Premix 2%
[0056] Appro...
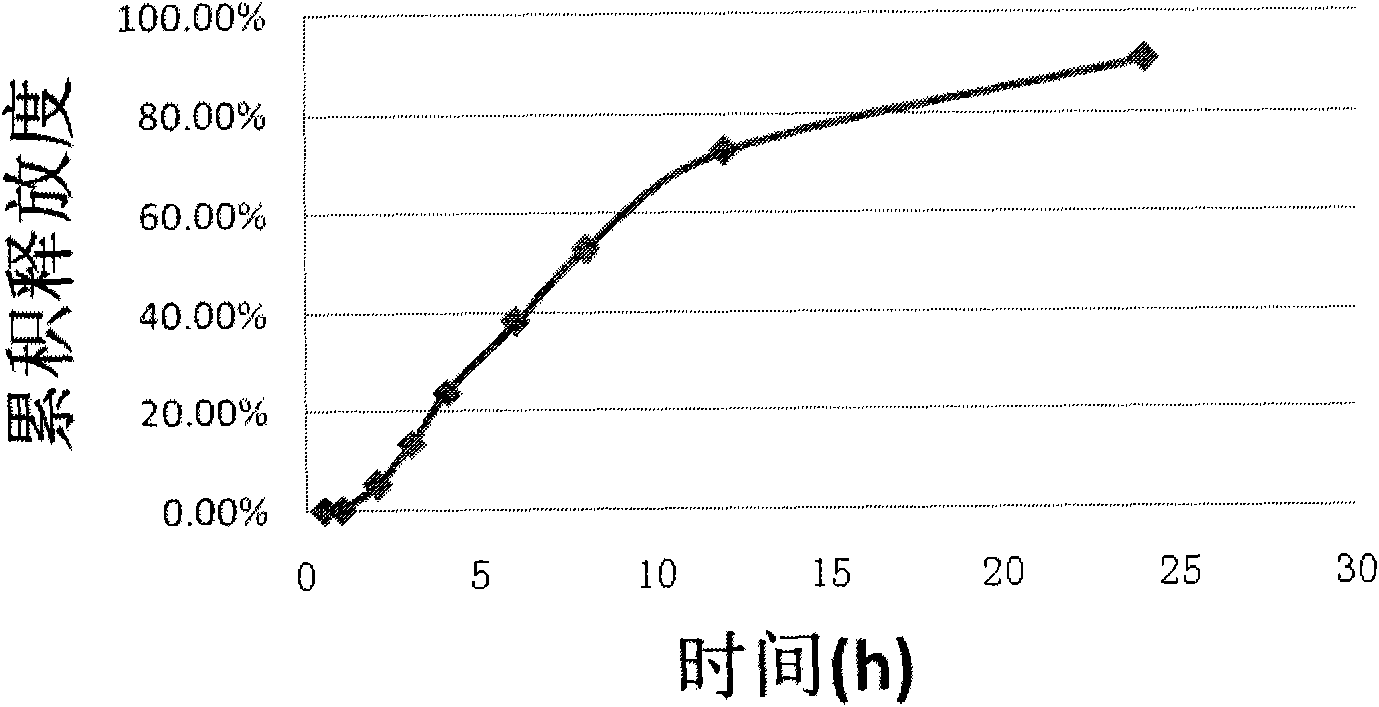
Embodiment 2
[0059] prescription:
[0060] Drug-containing layer (calculated by weight percentage of drug-containing layer)
[0061] Alamode 31.25%
[0062] Polyoxyethylene 62.5%
[0063] appropriate amount of lemon yellow
[0064] Sodium Lauryl Sulfate 4.38%
[0066] 5% PVA ethanol solution appropriate amount
[0067] Booster layer (calculated by weight percentage of booster layer)
[0068] Polyoxyethylene 62.5%
[0069] Sodium Chloride 25%
[0070] Hydroxypropyl Methyl Cellulose 3.12%
[0071] Polyvinylpyrrolidone k30 6.25%
[0072] Iron oxide red amount
[0073] Magnesium Stearate 1%
[0074] 8% PVA ethanol solution appropriate amount
[0075] Controlled release film (by weight percentage of tablet core)
[0076] Cellulose acetate 10.68%
[0077] Polyethylene glycol 4000 0.356%
[0078] Acetone: water appropriate amount
[0079] Protective layer: (by weight percentage of coated tablet)
[0080] Opadry Gastric Coating Premix 2%
[0081] Ap...
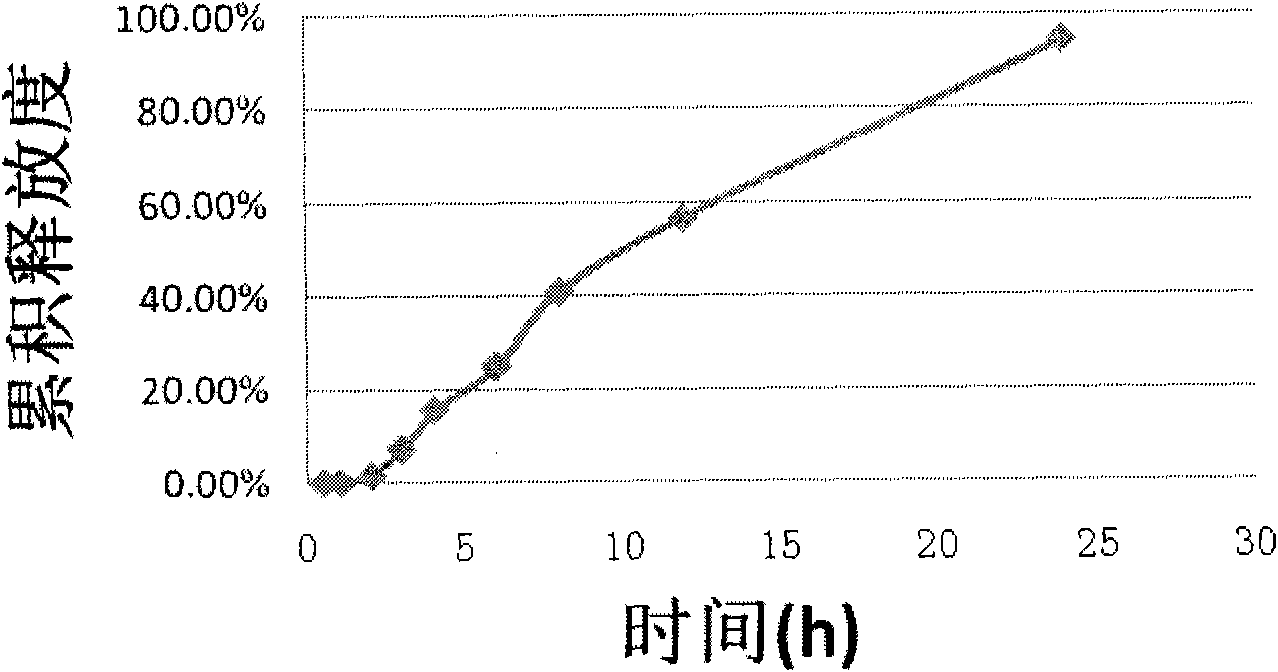
Embodiment 3
[0084] prescription:
[0085] Drug-containing layer (calculated by weight percentage of drug-containing layer)
[0086] Alamode 31.25%
[0087] Polyoxyethylene 62.5%
[0088] appropriate amount of lemon yellow
[0089] Sodium Lauryl Sulfate 4.38%
[0090] Magnesium Stearate 1%
[0091] 5% PVA ethanol solution appropriate amount
[0092] Booster layer (calculated by weight percentage of booster layer)
[0093] Polyoxyethylene 62.5%
[0094] Sodium Chloride 25%
[0095] Hydroxypropyl Methyl Cellulose 3.12%
[0096] Polyvinylpyrrolidone k30 6.25%
[0097] Iron oxide red amount
[0098] Magnesium Stearate 1%
[0099] 8% PVA ethanol solution appropriate amount
[0100] Controlled release film (by weight percentage of tablet core)
[0101] Cellulose acetate 13.8%
[0102] Macrogol 4000 0.46%
[0103] Acetone: water appropriate amount
[0104] Protective layer: (by weight percentage of coated tablet)
[0105] Opadry Gastric Coating Premix 2%
[0106] Appropriate amo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com