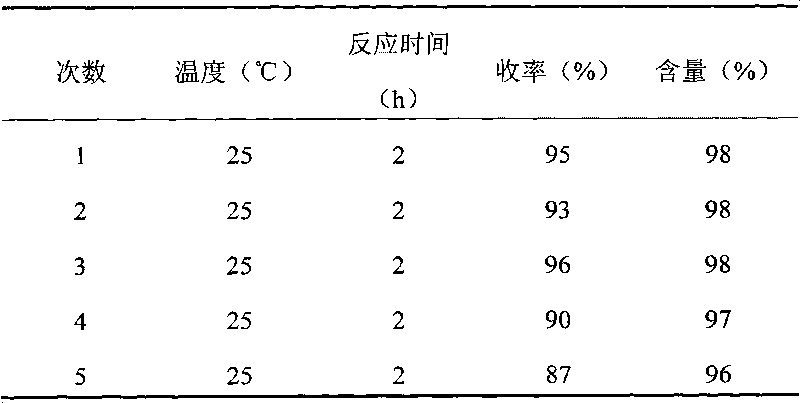
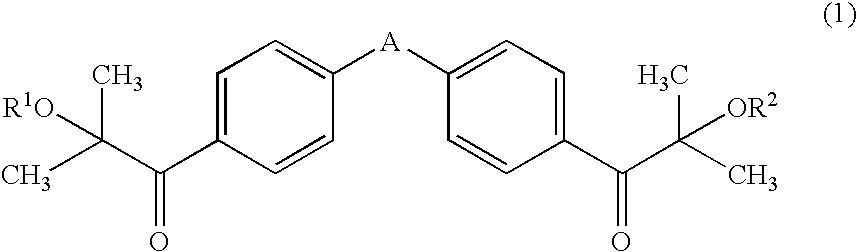
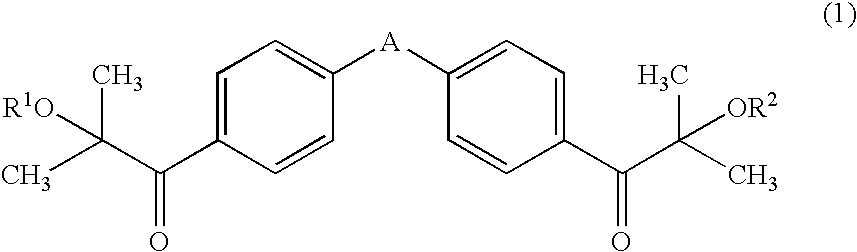
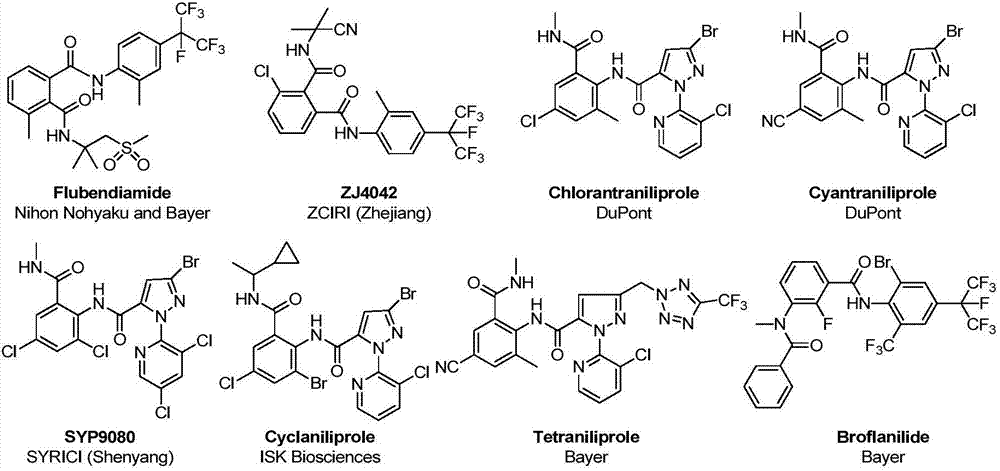
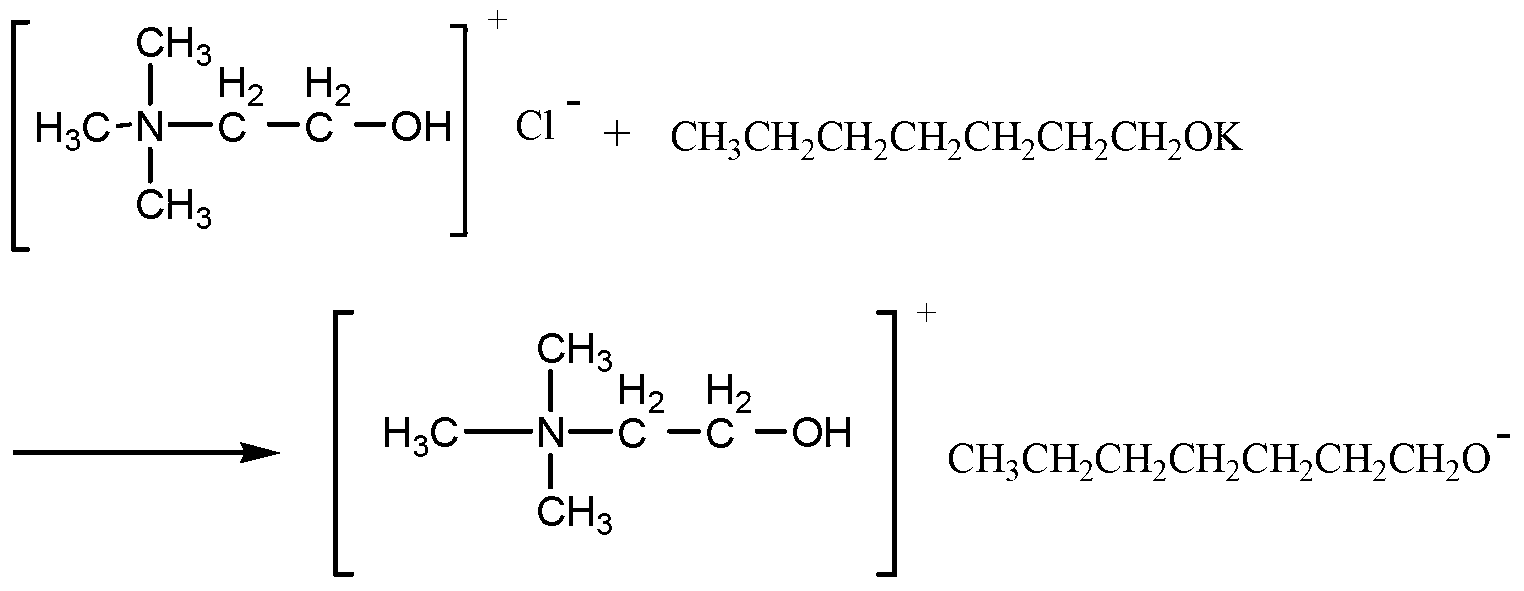
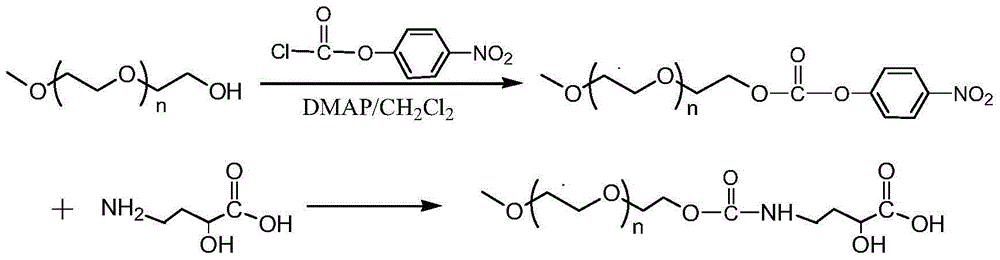
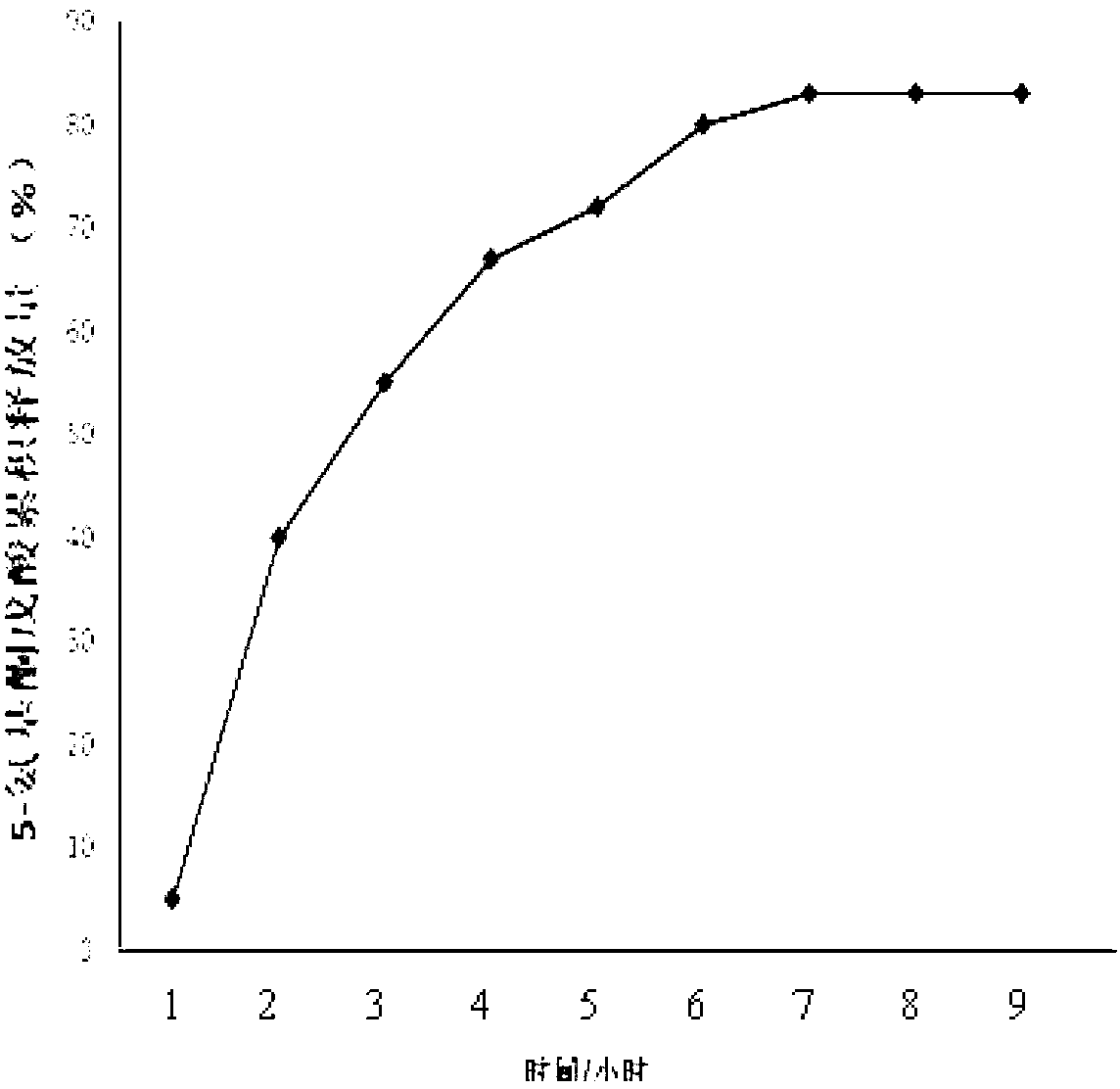
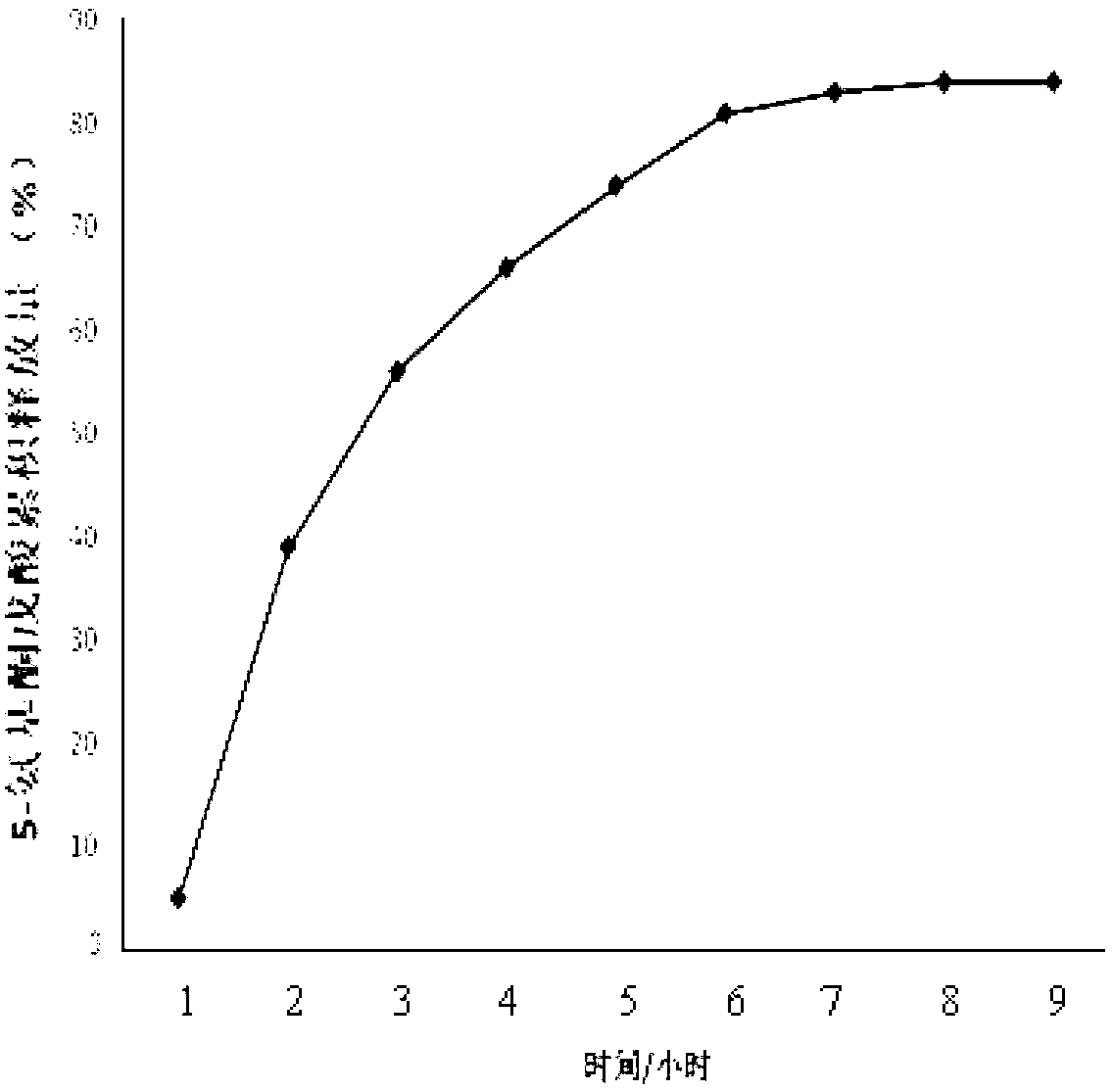
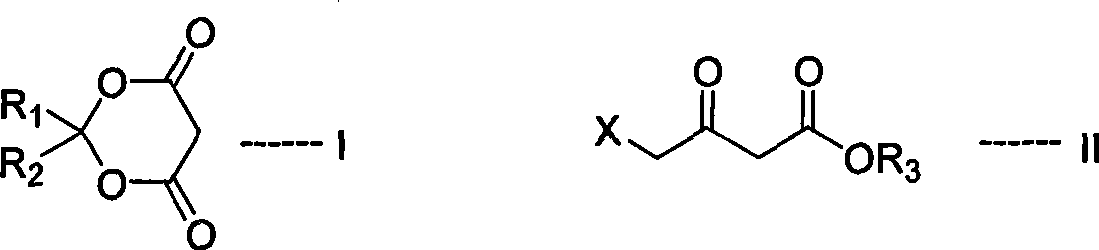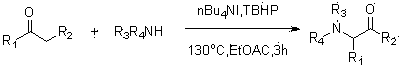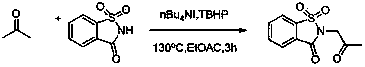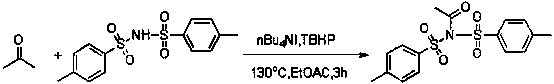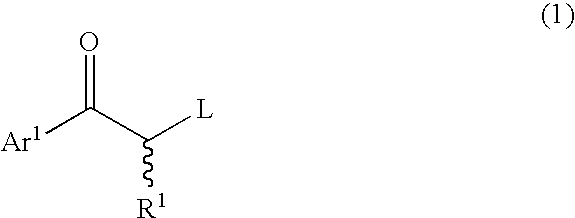Patents
Literature
172 results about "Aminoketone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
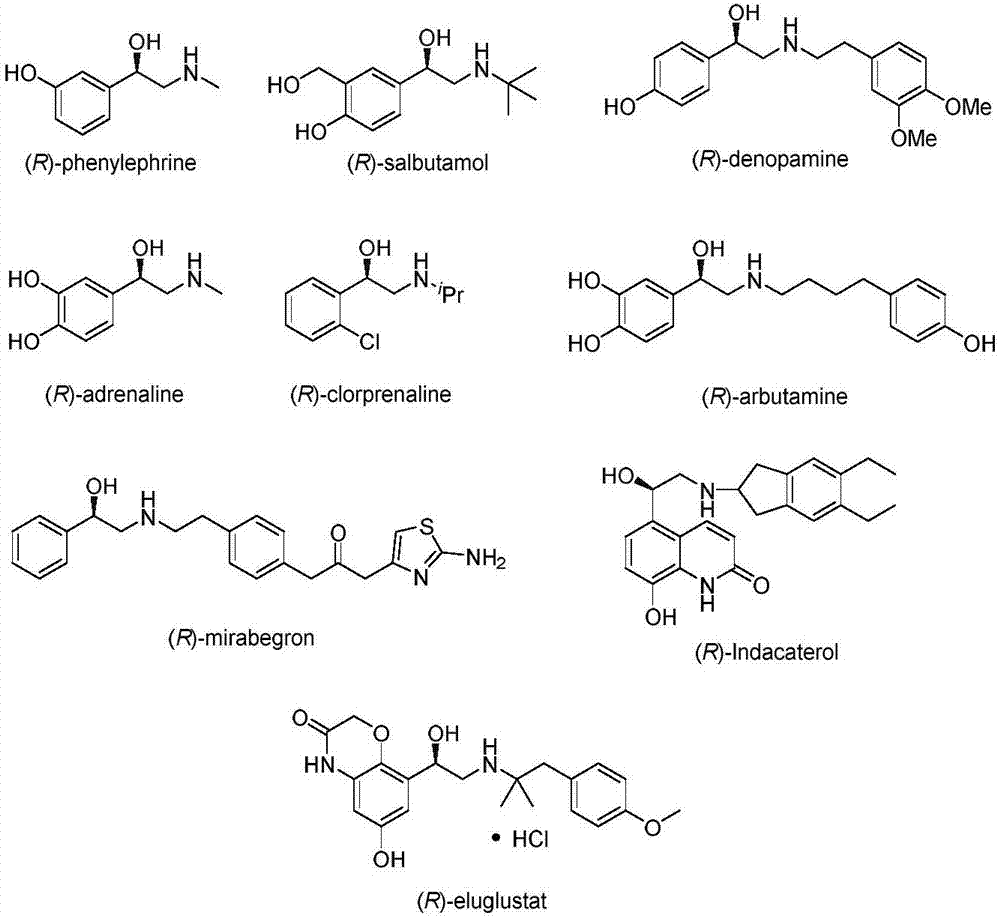
Aminoketones are compounds containing both a ketone group and an amine. Examples include cathinones, methadone, molindone, pimeclone, ferruginine, and tropinone.
Substituted amino ketone compounds
InactiveUS20050014946A1Organic active ingredientsOrganic compound preparationIGT - Impaired glucose toleranceNephrosis
The present invention relates to compounds of the general formula I B—(CH—R1)n—C(═X2)-D (I) and pharmaceutically acceptable salts thereof including stereoisomers, to the use of the compounds for the treatment of impaired glucose tolerance, glucosuria, hyperlipidaemia, metabolic acidosis, diabetes mellitus, diabetic neuropathy and nephropathy and of sequelae caused by diabetes mellitus in mammals.
Owner:PROSIDION LIMITED
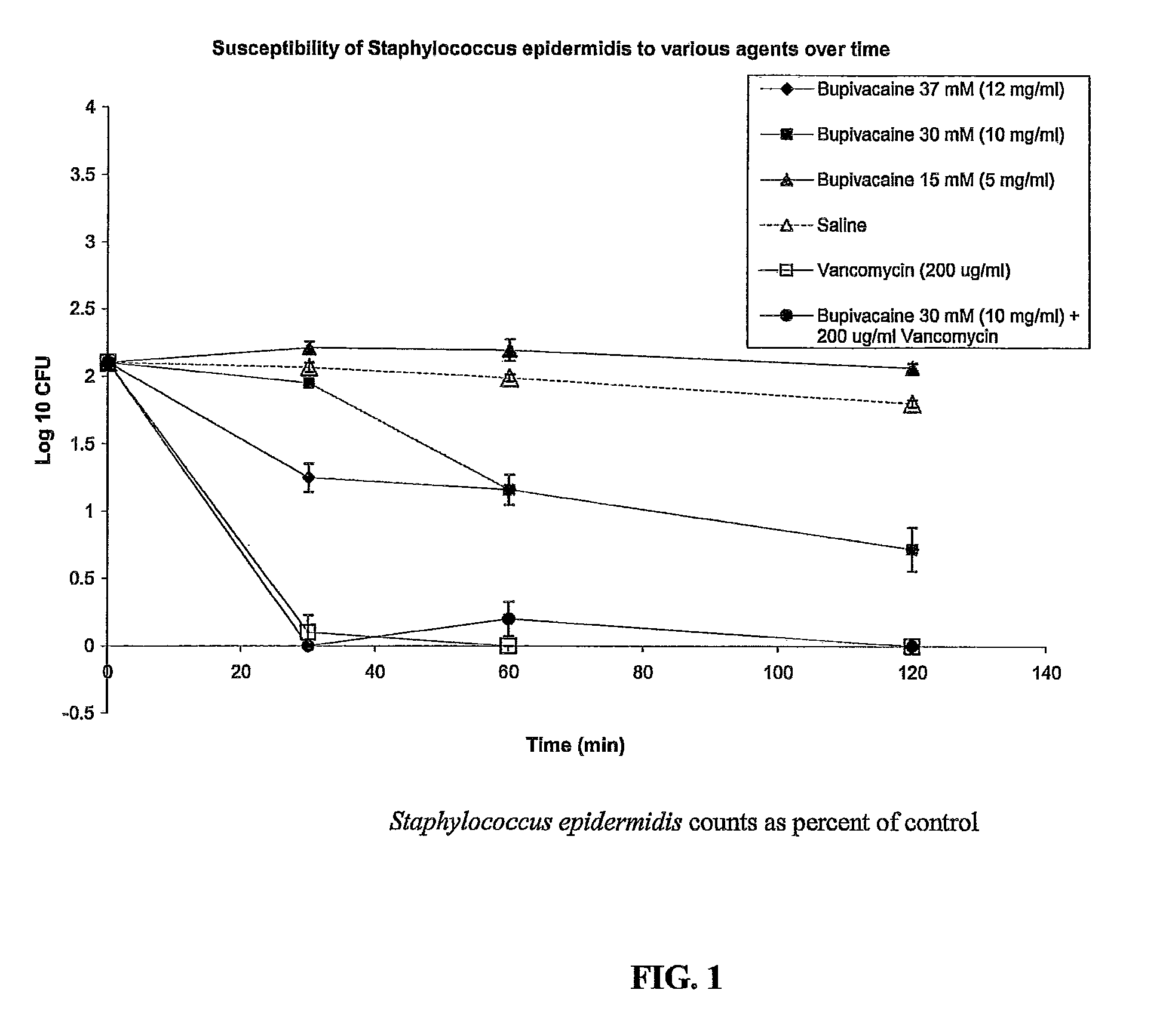
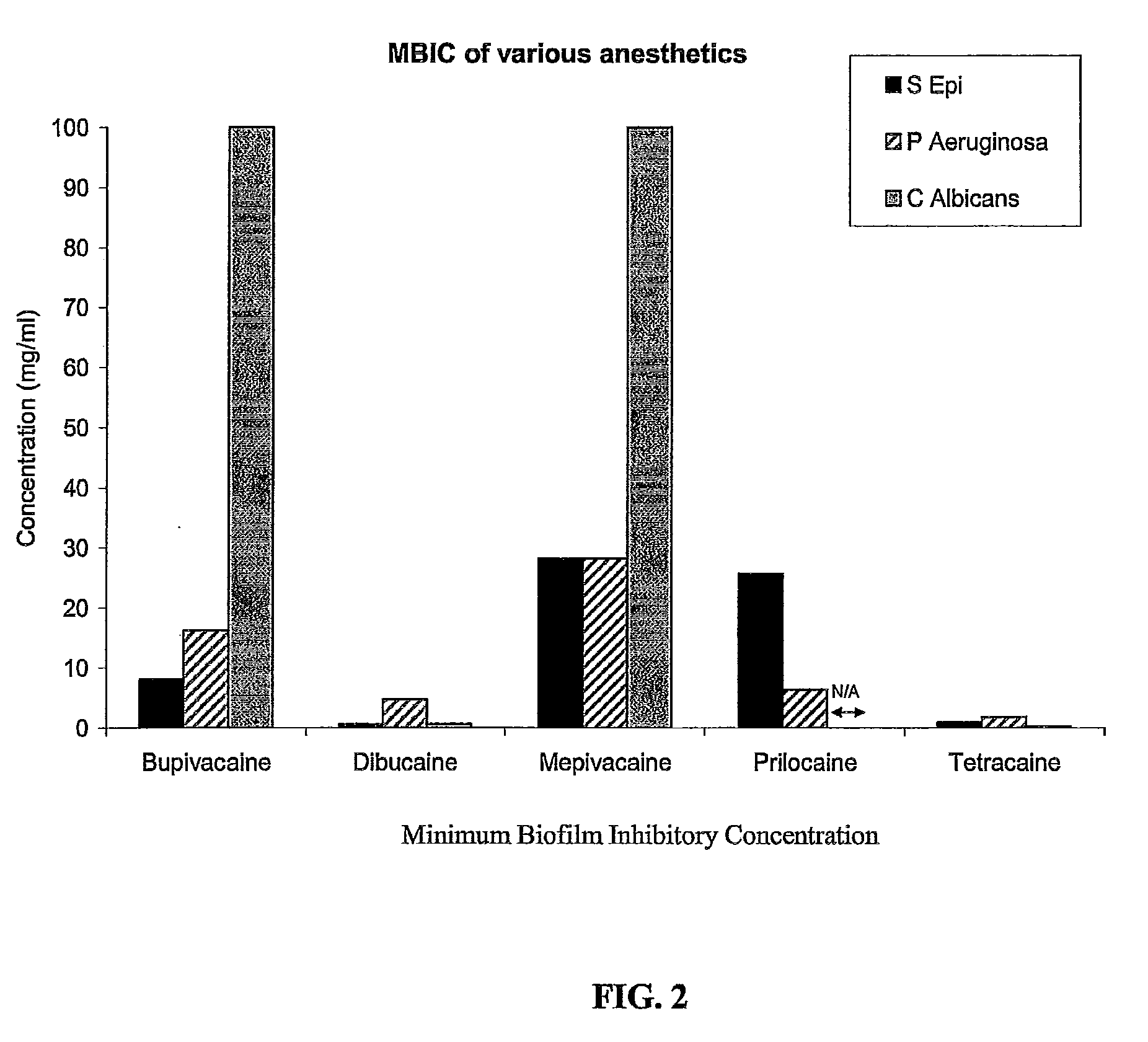
Catheter Locking Solution Having Antimicrobial and Anticoagulation Properties
ActiveUS20100318040A1Inhibiting antimicrobial activityPrevent coagulationAntibacterial agentsBiocideAnesthetic AgentAmino esters
The present invention includes a catheter locking solution having both antimicrobial and anticoagulant properties including a local anesthetic and a viscosifying agent. The local anesthetic of the present invention may be an amino amide; an amino ester; an aminoacylanilide; an aminoalkyl benzoate; an amino carbonate; an N-phenylamidine compound; an N-aminoalkyl amid; an aminoketone, or combinations and mixtures thereof. In a particular embodiment of the present invention, the local anesthetic is tetracaine or dibucaine.
Owner:BECTON DICKINSON & CO
Active Energy Ray-Curable Ink-Jet Printing Ink
ActiveUS20070289484A1Exhibit curabilityImprove curing effectImpression capsPhotomechanical apparatusHydrogen atomTrimethylsilyl
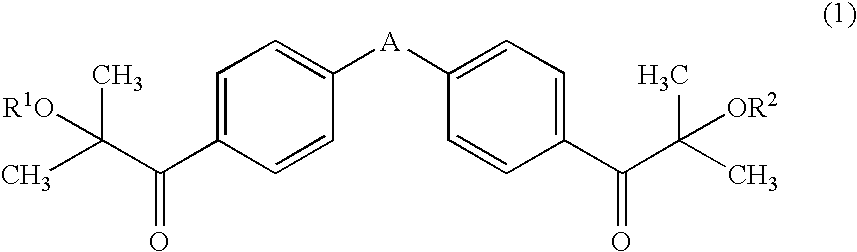
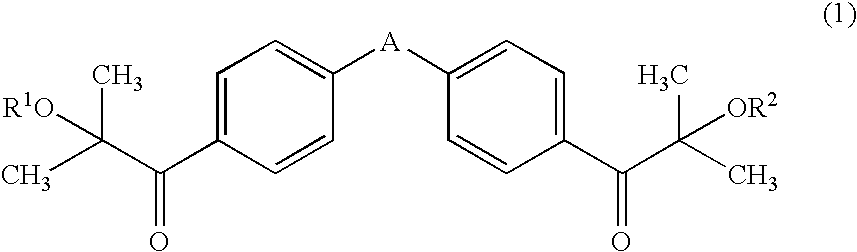
The object of the present invention is to provide an active energy ray-curable ink-jet printing ink, including: a coloring agent; a compound having an ethylenic double bond; and a photo-polymerization initiator, wherein the photo-polymerization initiator includes a compound represented by general formula (1): (wherein A represents any one of —O—, —CH2—, —CH(CH3)—, and —C(CH3)2—; and each of R1 and R2 independently represents a hydrogen atom, a methyl group, or a trimethylsilyl group), and an α-aminoketone-based compound and / or an acyl phosphine oxide-based compound, and 40% by mass or more of the compound represented by general formula (1) is included with respect to the total photo-polymerization initiator.
Owner:DAINIPPON INK & CHEM INC
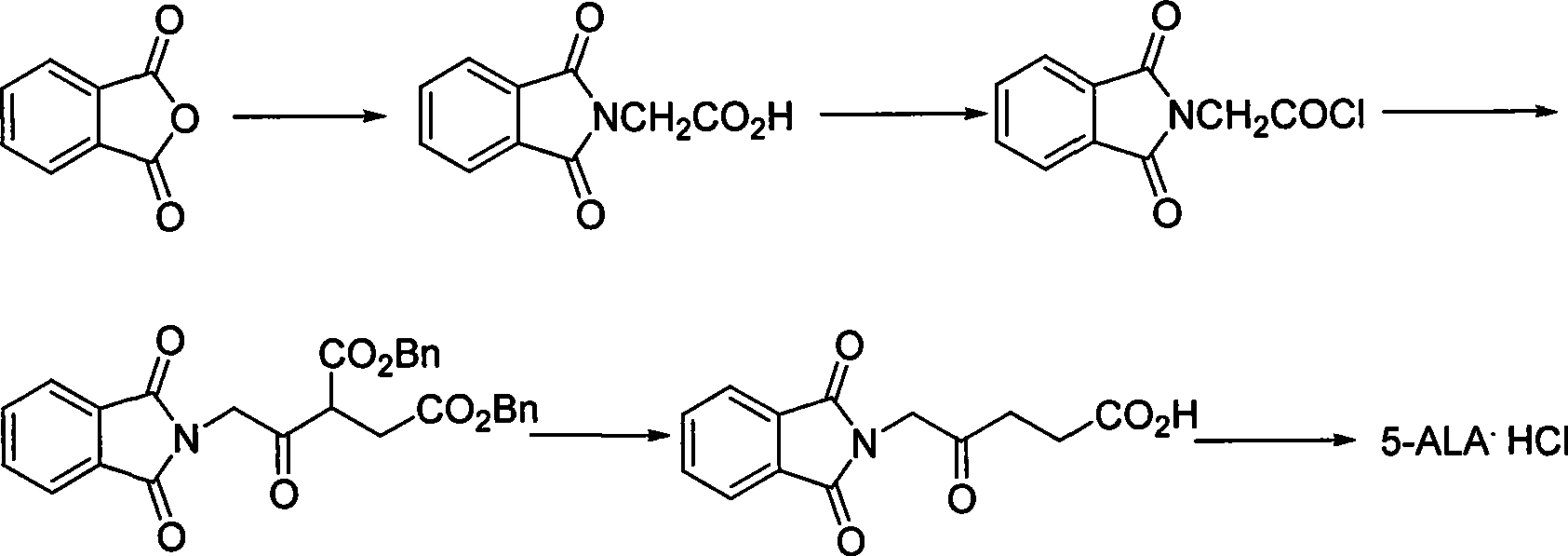
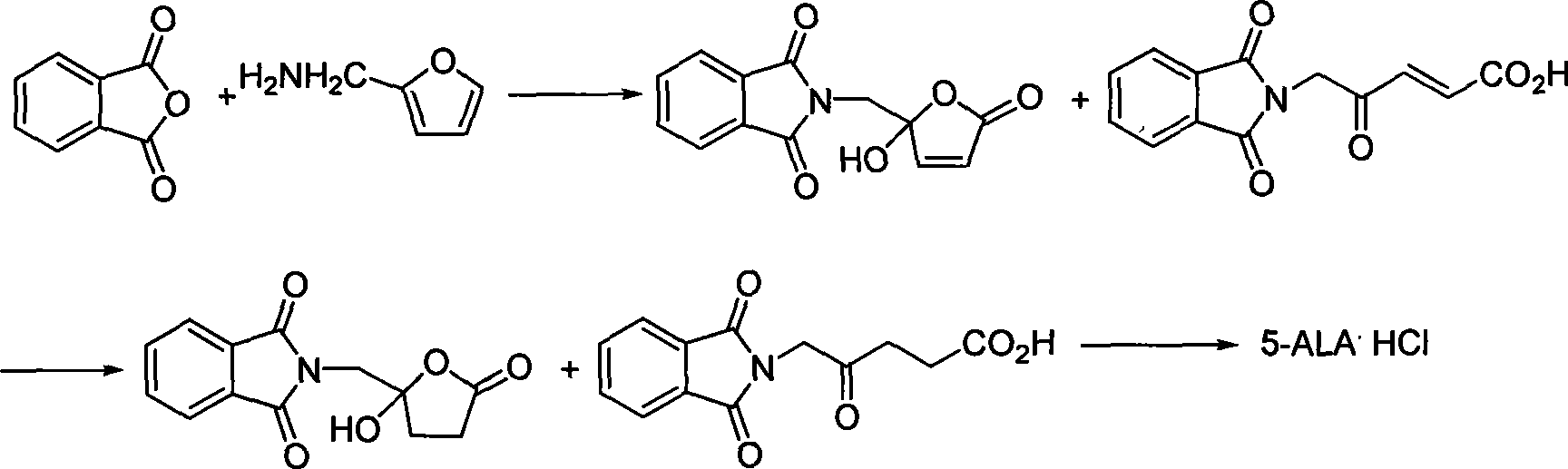
Process for synthesizing 5-aminovaleric acid hydrochloride
ActiveCN101462974AEasy to crystallize and purifyRaw materials are simpleOrganic compound preparationAmino-carboxyl compound preparationBenzyl groupAlkyl transfer
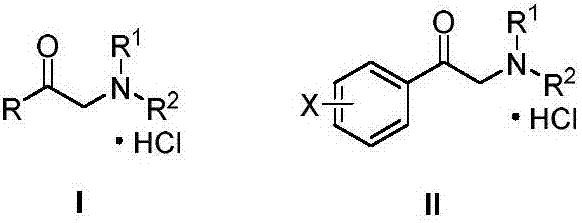
The invention relates to an efficient novel method for synthesizing photosensitizing agent 5-ketoamine valerate hydrochloride. The method uses compounds of formula (I) and formula (II). In the formulas, R1 and R2 represent hydrogen or C1-C4 alkyl or substituted benzyl; R3 represents the C1-C4 alkyl or the substituted benzyl; and X represents Cl, Br, OTs and OMs. High purity 5-ketoamine valerate hydrochloride is prepared after alkylation reaction, interesterification, hydroxylamination, reduction and hydrolysis decarboxylation finally.
Owner:FUJIAN BOTE CHEM PROD
Catheter locking solution having antimicrobial and anticoagulation properties
ActiveUS9248093B2Increase the viscosity of the solutionAvoid aspirationAntibacterial agentsBiocideAnesthetic AgentAmino esters
Owner:BECTON DICKINSON & CO
Ink composition
InactiveUS20120069108A1Curing can be defectivePrevent curingImpression capsInksWhitening AgentsKetone
The present invention provides an ink composition comprising at least a polymerizable compound, a photo-polymerization initiator and a polymerization accelerator, wherein the polymerizable compound comprises en N-vinyl compound, the photo-polymerization initiator comprises two or more compounds selected from the group consisting of bisacylphosphine oxides, monoacylphosphine oxides and α-amino ketones, and the polymerization accelerator comprises fine particles having a polymerizable functional group. The ink composition of the invention may be a transparent ink composition containing no coloring material. The ink composition of the invention may further contain a fluorescent whitening agent.
Owner:SEIKO EPSON CORP
Ink composition and inkjet recording method using the same
ActiveUS20080182031A1Efficient processImprove discharge stabilityInksPaints for electrolytic applicationsHydrogen atomPhosphine oxide
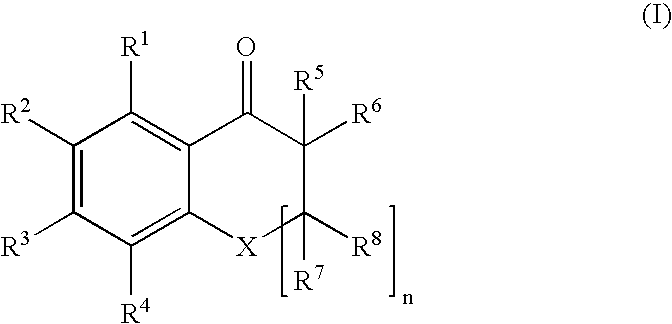
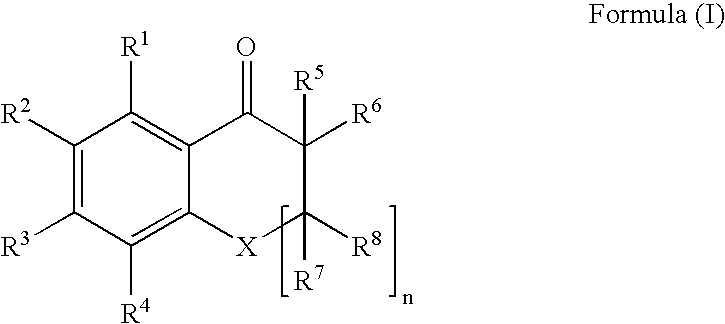
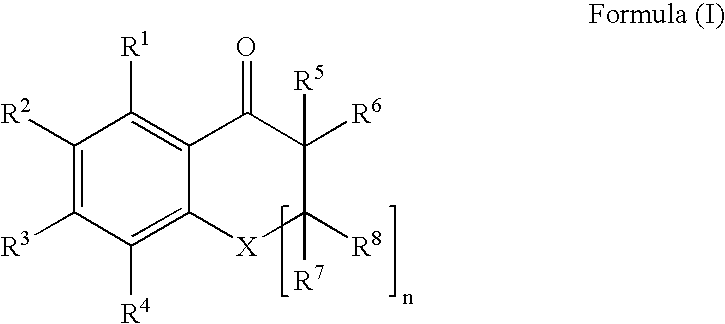
An ink composition of the present invention is disclosed, which contains (i) a sensitizing dye represented by the following Formula (I); (ii) at least one polymerization initiator selected from the group consisting of α-aminoketones and acyl phosphine oxides; and (iii) an ethylenically unsaturated bond-containing polymerizable compound. In Formula (I), X represents O, S or NR; n represents an integer of 0 or 1; R represents a hydrogen atom, an alkyl group or an acyl group; R1 to R8 each independently represent a hydrogen atom or a monovalent substituent; R1 and R2, R2 and R3, and R3 and R4 may be connected to each other to form a ring; and R5 or R6 may be connected to R7 or R8 to form an aliphatic ring but not to form an aromatic ring.
Owner:FUJIFILM CORP
Novel method for preparing beta-aminoketone, ester, nitrile and amide derivatives through catalysis of functional ionic liquid
InactiveCN101723771AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSolvent freeSolvent
The invention relates to a method for generating beta-aminoketone, ester, nitrile and amide derivatives by performing aza-Michael addition on amine substances and electron-deficient alkenes through an efficient environment-friendly catalyst under solvent-free mild (room temperature) reaction conditions. The method comprises the steps of taking ionic liquid as the catalyst, subjecting amine substances and electron-deficient alkenes to aza-Michael addition at room temperature under normal pressure and obtaining corresponding beta-aminoketone, ester, nitrile and amide derivatives. The ionic liquid is repeatedly used five times, and reaction yield does not obviously drop. The method has the advantages of simple operation, high yield, good using repeatability of the catalytic reaction system, mild reaction conditions and good prospects for industrialization.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY +1
Active energy ray-curable ink-jet printing ink
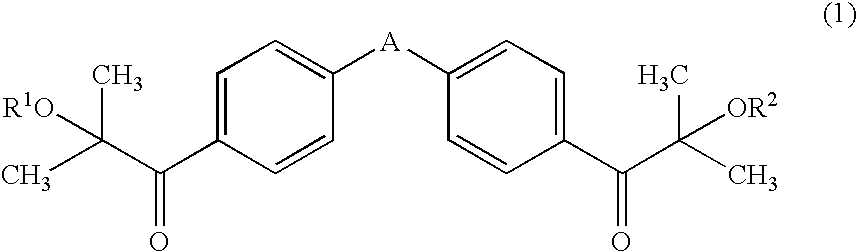
The object of the present invention is to provide an active energy ray-curable ink-jet printing ink, including: a coloring agent; a compound having an ethylenic double bond; and a photo-polymerization initiator, wherein the photo-polymerization initiator includes a compound represented by general formula (1):(wherein A represents any one of —O—, —CH2—, —CH(CH3)—, and —C(CH3)2—; and each of R1 and R2 independently represents a hydrogen atom, a methyl group, or a trimethylsilyl group), and an α-aminoketone-based compound and / or an acyl phosphine oxide-based compound, and 40% by mass or more of the compound represented by general formula (1) is included with respect to the total photo-polymerization initiator.
Owner:DAINIPPON INK & CHEM INC
Diphenylamino ketone derivatives as MEK inhibitors
The present invention relates to diphenylamino ketone derivatives, pharmaceutical compositions and methods of use thereof.
Owner:PFIZER INC
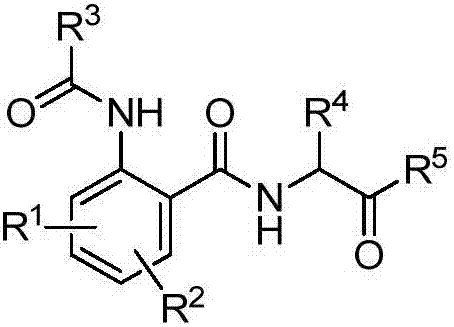
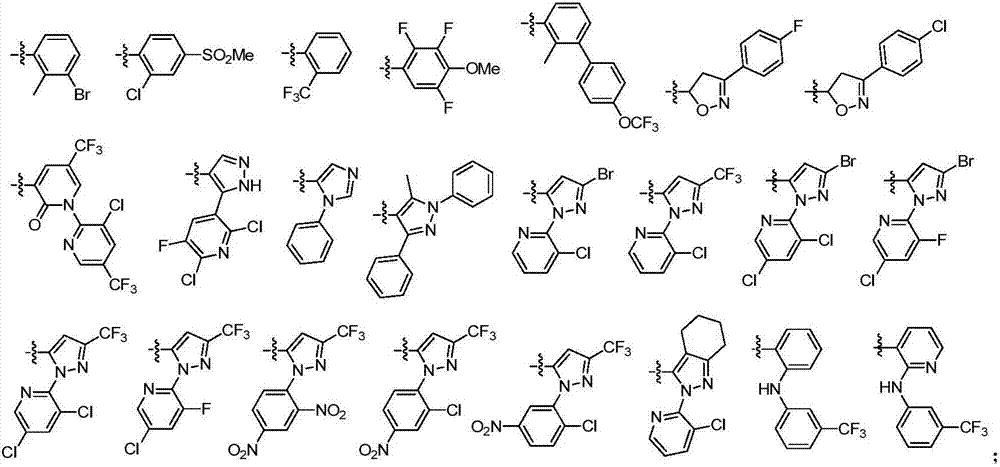
Amide derivative containing alpha-aminoketone structure and preparation method and application thereof
ActiveCN107056747AEasy to manufactureRaw materials are easy to getBiocideOrganic chemistryWater dispersibleStructural formula
The invention discloses an amide derivative containing an alpha-aminoketone structure and a preparation method and application thereof. A structural formula of the amide derivative is shown in the attached figure. The preparation method comprises the following step of using substituted o-aminobenzoic acid and substituted carboxylic acid R3COOH as initial raw materials, and performing a series of reactions, so as to obtain the amide derivative. When the amide derivative is used for preparing an insecticide, the amide derivative containing the alpha-aminoketone structure is dissolved into a thinner, so as to obtain the insecticide; the weight percentage of the amide derivative containing the alpha-aminoketone structure in the insecticide is 0.001 to 99.99%. During specific application, the insecticide is a conventional preparation, such as a water emulsion, a suspension agent, wettable powder and a water dispersible granule.
Owner:HUBEI BIOPESTICIDE ENG RES CENT
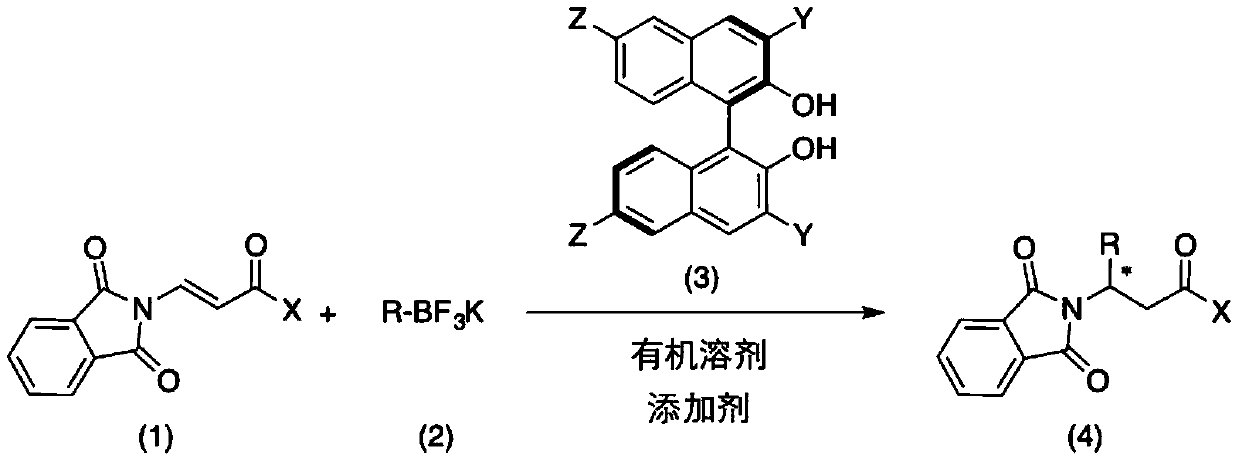
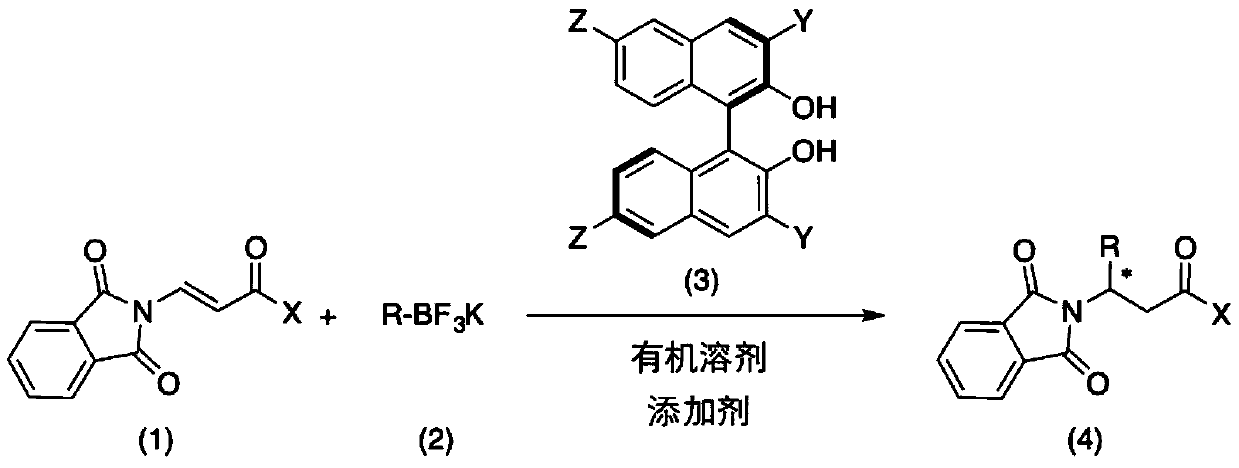
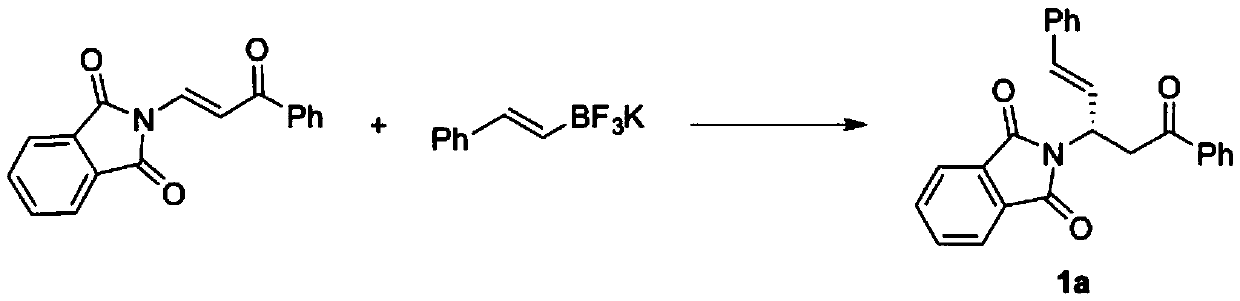
Method for catalytically asymmetrically synthesizing chiral beta-aminoketone derivative
ActiveCN109748841AReduce usageLower reaction costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventMetal catalyst
The invention discloses a method for catalytically asymmetrically synthesizing a chiral beta-aminoketone derivative. The method includes taking a chiral binaphthol derivative shown in a formula (3) asa catalyst; and in the presence of an organic solvent and an additive, preforming, by potassium trifluoroborate shown in a formula (2), an asymmetric 1,4-addition reaction on a beta-phthalimide acrylone compound shown in a formula (1) to generate the beta-aminoketone derivative containing one chiral center shown in a formula (4), wherein the reaction formula is shown in the specification. The invention discloses a new chiral catalyst of a polyfluoroaphthol skeleton, adopts nonmetallic catalysis, avoids the use of metal catalysts from the source, and reduces the reaction cost. The yield and enantioselectivity of the target product are high, the reaction conditions are simple and mild, and a method for preparing multiple chiral beta-aminoketone derivatives is provided, so that the method has better application prospect and social value.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing beta-aminoketone, ester and nitrile amide derivatives
InactiveCN103304516AImprove solubilityAdjustable molecular structureOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsRoom temperatureIonic liquid
The invention discloses a method for preparing beta-aminoketone, ester and nitrile amide derivatives. The method comprises the steps of: by taking choline chloride functional ionic liquid as a catalyst, carrying out aza-Michael addition reaction on an amine substance and electron-deficient olefins at room temperature and normal pressure, so as to obtain the beta-aminoketone, ester, nitrile amide derivatives. The choline chloride functional ionic liquid can be repeatedly utilized for a plurality of times. The method is simple to operate, high in yield, good in reusability of a catalytic reaction system and mild in reaction condition, and has a good industrial prospect.
Owner:TAIZHOU UNIV
Semi-solid compositions and pharmaceutical products
This invention relates to semi-solid compositions and semi-solid pharmaceutical products for use in the photodynamic treatment (PDT) of cancer, pre-cancerous conditions and non-cancerous conditions in the female reproductive system, the anus and the penis, preferably for use in PDT of endometrial, cervical, vulvar, vaginal, anal and penile dysplasia and HPV infections of the uterus, cervix, the vulva, the vagina, the anus and the penis. The semi-solid compositions and pharmaceutical products comprise an active ingredient which is 5-aminolevulinic acid (5-ALA) or a precursor or derivative of 5-ALA or pharmaceutically acceptable salts thereof. The invention relates further to methods of PDT of cancer, pre-cancerous conditions and non-cancerous conditions in the female reproductive system, the anus and the penis, wherein said semi-solid compositions and pharmaceutical products are used.
Owner:PHOTOCURE
Method for efficiently synthesizing chiral 1,2-amino alcohol by catalyzing alpha-aminoketone through Ir/f-amphox
ActiveCN107021884AHigh activityEasy to synthesizeOrganic compound preparationOrganic chemistry methodsAlcoholEnantio selectivity
The invention discloses a method for efficiently synthesizing chiral 1,2-amino alcohol by catalyzing alpha-aminoketone through Ir / f-amphox. A ligand f-amphox used by the method can be more easily synthesized; the reaction has the characteristics of enantioselectivity, high yield and high TON (turn over number); most substrates achieve the conversion rate being 99 percent or higher and the ee value being 99 percent or higher under the condition of the catalyst dosage being 0.002 mol% (S / C=50000); the highest TON reaches 500000 and is the maximum value reported at the present. The chiral 1,2-amino alcohol can be successfully used for the synthesis of a series of important medicines; important significance is realized on medicine industrial production.
Owner:WUHAN CATALYS TECH CO LTD +1
Preparation method for alpha-amino ketone derivative
InactiveCN102584620AImprove conversion rateReduce pollutionOrganic compound preparationCarboxylic acid amides preparationArylCarboxyl radical
The invention discloses a preparation method for an alpha-amino ketone derivative as represented by formula II. The derivative is prepared by subjecting a compound as represented by formula I to a reaction in an organic solvent under the action of trichloroisocyanuric acid and an oxidation catalyst or subjecting the compound as represented by formula I to a reaction in an organic solvent with a pH value of 8 to 11 under the action of an oxidizing agent, an oxidation catalyst, a pH conditioning agent and a phase-transfer catalyst, wherein, R1, R2 and R3 are, independently, lower alkyl groups, aryl groups, aryl-substituted lower alkyl groups, lower alkoxy groups, aryl-substituted lower alkoxy groups, carboxyl-substituted lower alkyl groups or carbonyl groups connected with lower alkyl groups, and R4 is a carbonyl group connected with a lower alkyl group, a carbonyl group connected with an aryl group or a carbonyl group connected with a phenyl-substituted lower alkyl group. The preparation method provided in the invention has the advantages of cheap and easily available raw materials, mild reaction conditions, small pollution to the environment and a high conversion rate of the raw material and is beneficial for industrial large-scale production.
Owner:SHANGHAI INST OF PHARMA IND
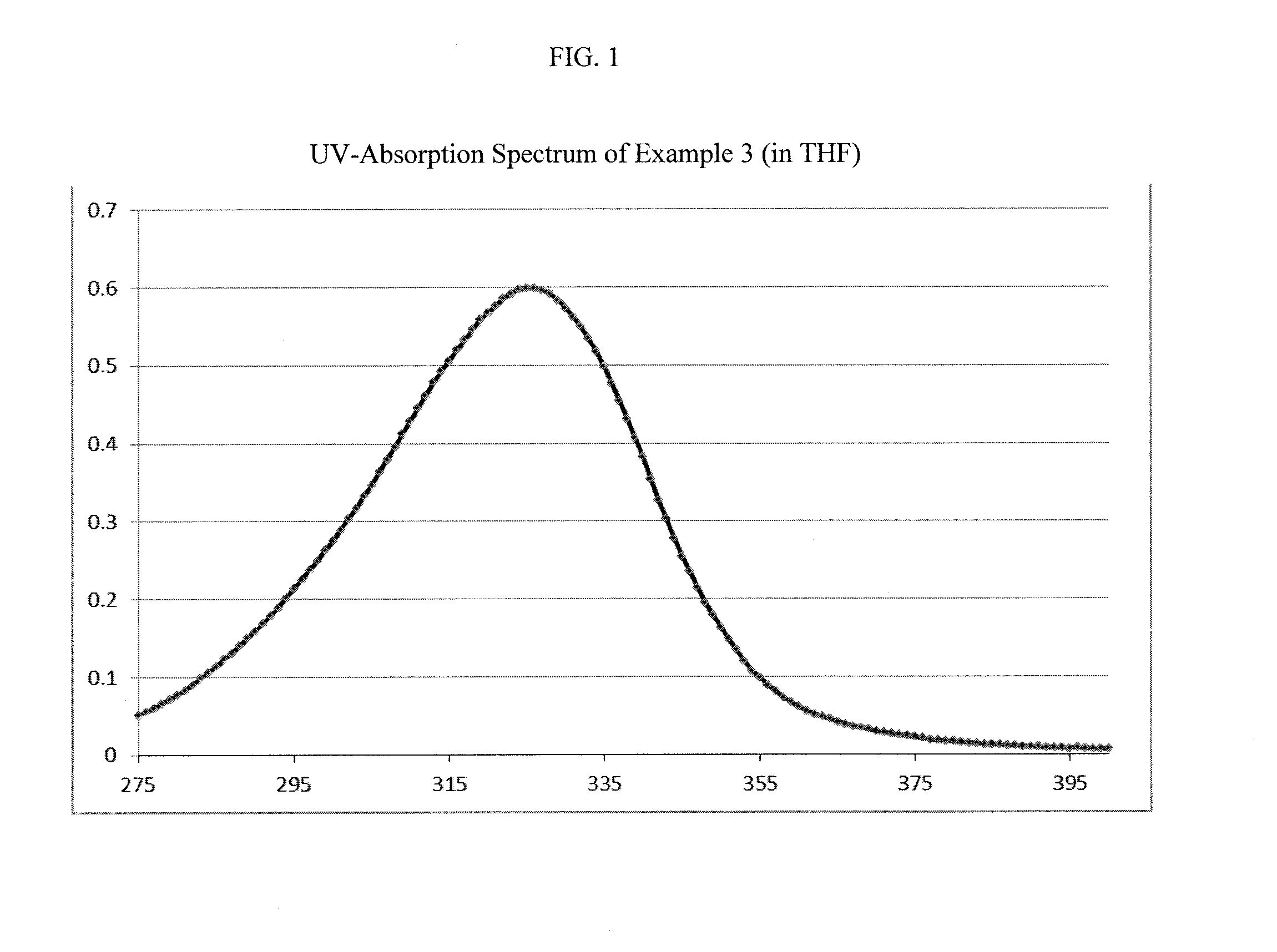
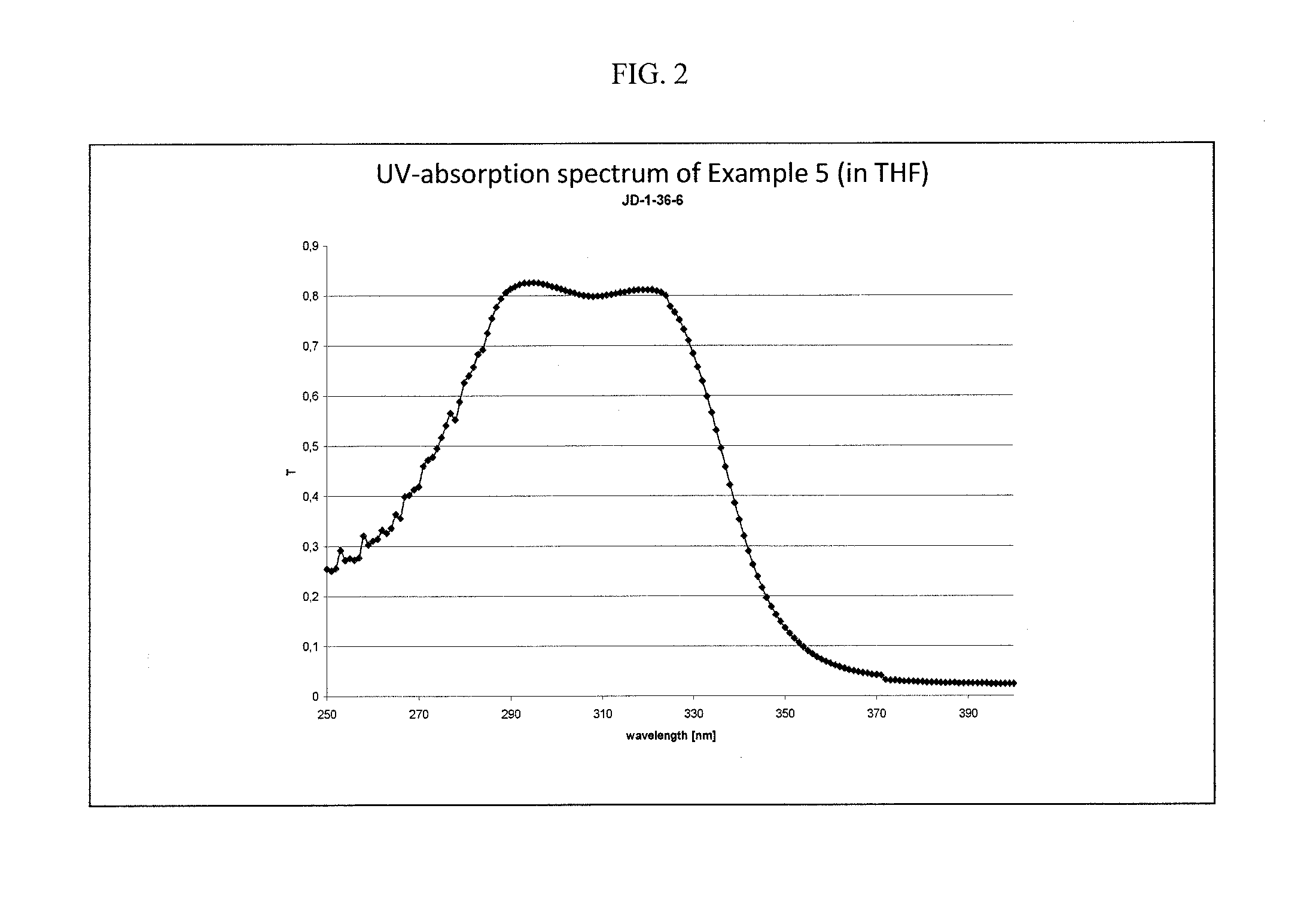
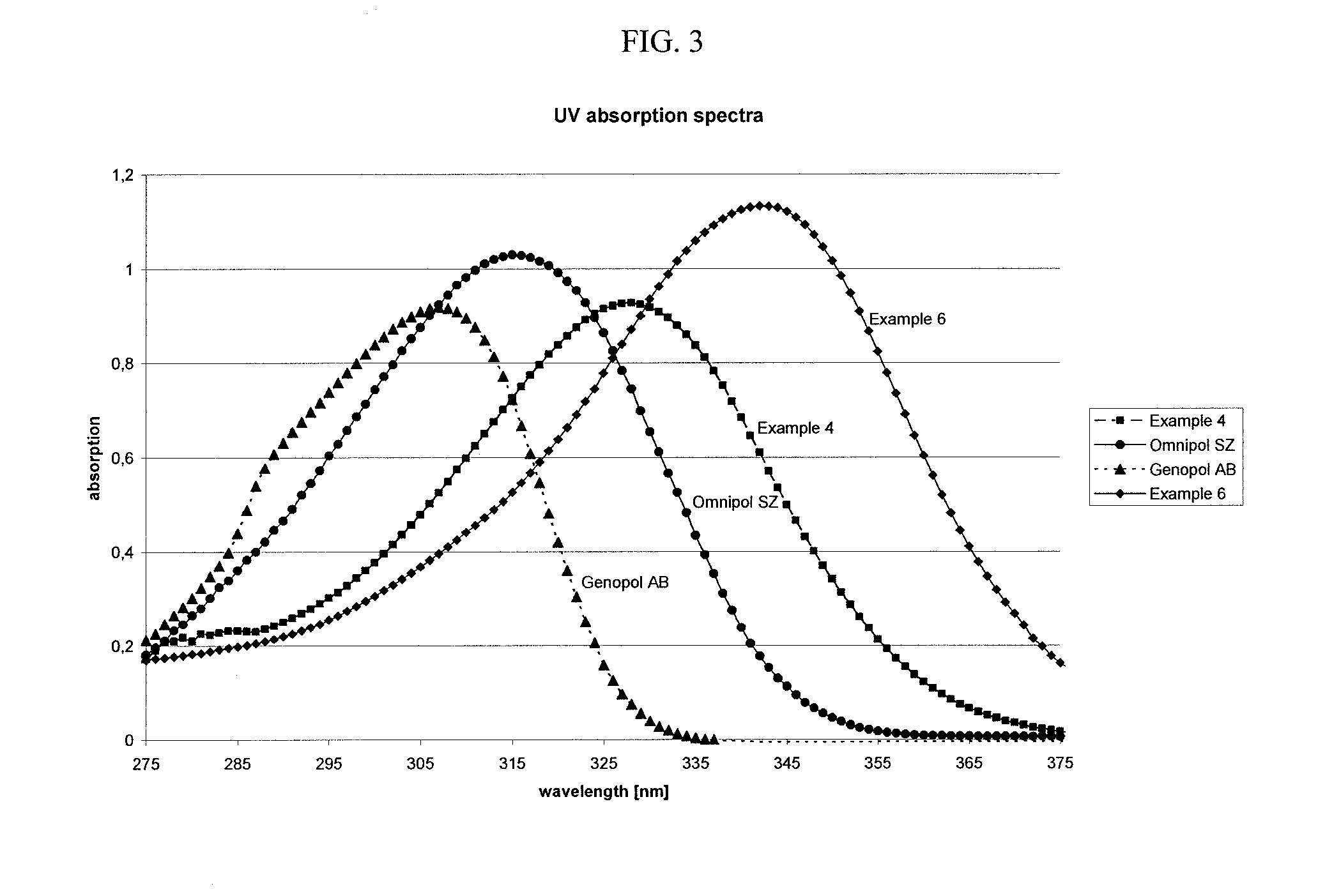
Novel aminoketones photoinitiator and application in UV-LED photocuring system
ActiveCN104974053AGood electron delocalizationStrong intramolecular electron transfer propertiesOrganic compound preparationSulfide preparationElectron delocalizationHigh energy
The invention provides a novel aminoketones photoinitiator suitable for UV-LED light source curing. The novel aminoketones photoinitiator can be higher in adsorption in a long-wavelength region (365-395nm), is suitable for UV-LED light source curing, and overcomes the defects of high energy consumption and heavy pollution in traditional curing. The novel aminoketones photoinitiator is good in eectron delocalizability, has high intramolecular electron transfer performance and excellent photoelectric property, is higher in adsorption in the range of 365nm to 395nm in the long-wavelength region, is suitable for a high-power UV-LED ultraviolet photocuring system, and has considerable advantages compared with the photoinitiator in the prior art.
Owner:TIANJIN JIURI NEW MATERIALS CO LTD
UV photocuring composition containing aminoketone photoinitiator
InactiveCN109762397AHigh priming activityShort curing timeInksPolyurea/polyurethane coatingsSurface layerCoating system
The invention provides a UV photocuring composition containing an aminoketone photoinitiator. The composition is suitable for a photocuring system, mainly suitable for a UV photocuring system, and particularly suitable for a UV photocuring ink and coating system; the UV photocuring composition containing the aminoketone photoinitiator is high in activity and can be subjected to radiation curing through a high-pressure mercury lamp and a UV-LED; surface layer curing of a coating can be achieved, and deep layer curing can also be achieved; and the UV photocuring composition has wide applicationprospects.
Owner:CHANGSHANG NEWSUN CHEM IND
Protein polypeptide drug N-terminal fixed-point polyethylene glycol modification method
ActiveCN104926920AConvenient sourceMild conditionsPeptide preparation methodsAlpha hydroxy acidHigh activity
The invention discloses a protein polypeptide drug N-terminal fixed-point polyethylene glycol modification method. The method comprises the steps that 1, PEG terminal hydroxyl groups are converted into groups with higher activity to obtain a PEG active intermediate, and the PEG active intermediate and a small molecule compound of a hydroxyphenylacetic acid or alpha-hydroxy acid or beta-amino alcohol or alpha-aminoketones structure react to obtain a polyethylene glycol intermediate; 2, the polyethylene glycol intermediate is oxidized by an oxidizing agent to be a polyethylene glycol aldehyde derivative with active aldehyde groups at the terminal, then the polyethylene glycol aldehyde derivative is used for protein polypeptide drug N-terminal fixed-point polyethylene glycol modification, pegylation protein polypeptide drugs of a stable structure can be obtained through the reductive amination effect of a reducing agent, and the polyethylene glycol activity modifying agent preparation and decoration of the polyethylene glycol activity modifying agent for the protein polypeptide drugs need to be tightly connected. According to the protein polypeptide drug N-terminal fixed-point polyethylene glycol modification method, the problem that many polyethylene glycol aldehyde derivatives are likely to lose efficacy due to improper storage can be avoided, the period is short, and the cost is low.
Owner:ZHEJIANG PHARMA COLLEGE
Pleuromutilin compound, preparation method of pleuromutilin compound, polymorphism and preparation method of polymorphism
InactiveCN105399684AThe synthesis process is simpleThe post-processing method is simpleAntibacterial agentsOrganic chemistry methodsEscherichia coliThio-
The invention discloses a preparation method of a pleuromutilin compound, a polymorphism and a preparation method of the polymorphism. The pleuromutilin compound comprises a 14-O-[(4-amino-6-hydroxy-pyrimidine-2-yl) thio acetyl] matrix shown in a structural formula (i) and / or a 14-O-[(4-amino-6-one-pyrimidine-2-yl) thio acetyl] matrix shown in a structural formula (ii). The novel pleuromutilin compound disclosed by the invention has obvious inhibiting effects on multiple drug resistant bacteria such as methicillin resistant staphylococcus aureus and staphylococcus epidermidis as well as common pathogenic bacteria such as escherichia coli and streptococcus mastitidis in veterinary clinics, so that the pleuromutilin compound can be applied to preparation of antibacterial drugs and particularly anti-drug resistant bacterium drugs.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Method for synthesizing alpha-amino ketone from ketone and imine
InactiveCN103626714AAchieving direct aminationThe experimental steps are simpleSulfonic acid amide preparationOrganic synthesisKetone
The invention discloses a method for synthesizing alpha-amino ketone from ketone and imine, and belongs to the technical field of chemical organic synthesis. The method comprises the following steps: adding imine into an organic solvent, sufficiently dissolving so as to obtain an imine solution, further respectively adding ketone, a catalyst tetrabutyl ammonium iodide and an oxidant tert-butyl hydroperoxide into the solution, sealing up a tube opening, heating to be 130 DEG C, stirring for 3 hours, cooling down the system to be the room temperature after the reaction is ended, extracting by using dichloromethane and removing the solvent of on organic phase so as to obtain alpha-amino ketone. By adopting the method, defects in the conventional method for synthesizing alpha-amino ketone by electrophilic amination and nucleophilic amination are overcome, direct amination of alpha-position of a simple ketone compound such as acetone is achieved, a C-N bond is generated through oxidative coupling reaction between a N-H bond and a C-H bond at one step, and the alpha-position of a ketone substrate does not need to be prefunctionalized, so that the experiment steps are simple, the condition is gentle, the reagents are cheap, and the range of the substrate is wide; the method is applicable to industrial production.
Owner:NORTHEAST NORMAL UNIVERSITY
5-aminolevulinic acid NANO particle as well as preparation method and device thereof
InactiveCN102697731AFully lysedStable in natureAntibacterial agentsPowder deliveryAmino-Levulinic AcidAminolevulinic Acid Hydrochloride
The invention discloses a 5-aminolevulinic acid nano particle as well as a preparation method and a device thereof. The 5-aminolevulinic acid nano particle comprises therapeutically effective amount of 5-aminolevulinic acid, ion chelating agent and biodegradable polymer. The 5-aminolevulinic acid nano particle provided by the invention is stable in property, high in targeting selectivity, high in cell absorption rate, weakened in photo-bleaching phenomenon, and reinforced in photodynamic reaction, and is capable of enhancing tissue penetration depth combined with injection or micro-needle drug administration, so the 5-aminolevulinic acid nano particle has high application value.
Owner:HUADONG HOSPITAL +1
Plasmids and utilization thereof
Owner:DAIICHI FINE CHEM CO LTD
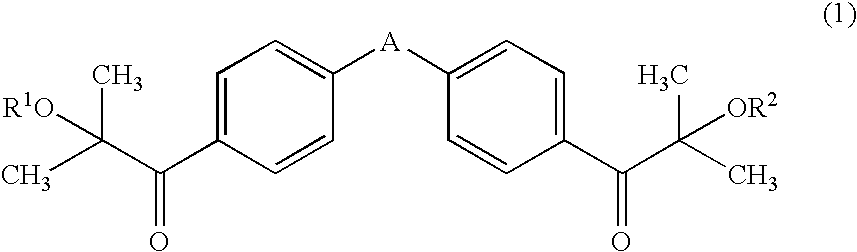
Oligomeric aminoketones and their use as photoinitiators
The invention relates to photoactive oligomeric aminoketones of general Formulae I and II, for compositions and inks curable with ultraviolet (UV) light. The oligomeric aminoketones of the general Formula I are made by condensation of aminoketones with aldehydes, and aminoketones of the general Formula II are made by acylation of aromatic amines with phthalic anhydride, followed by esterification with a polyol. The aminoketones provide good curability of energy curable coatings and inks with UV-A light, and are proposed for photoinitiator systems for low migration inks.
Owner:SUN CHEM CORP
Solid compositions comprising 5-aminolevulinic acid
InactiveCN102802612AOrganic active ingredientsIn-vivo radioactive preparationsAmino-Levulinic AcidLower Gastrointestinal Tract
This invention relates to solid compositions and solid pharmaceutical products for use in methods of photodynamic diagnosis of cancer, pre-cancerous and non-cancerous conditions in the lower part of the gastrointestinal system. The solid pharmaceutical compositions and pharmaceutical products comprise an active ingredient which is 5-aminolevulinic acid (5-ALA) or a precursor or derivative of 5-ALA or pharmaceutically acceptable salts thereof. The invention relates further to methods of photodynamic diagnosis of cancer, pre-cancerous and non-cancerous conditions of the lower gastrointestinal tract, wherein the solid pharmaceutical compositions and pharmaceutical products are used.
Owner:PHOTOCURE
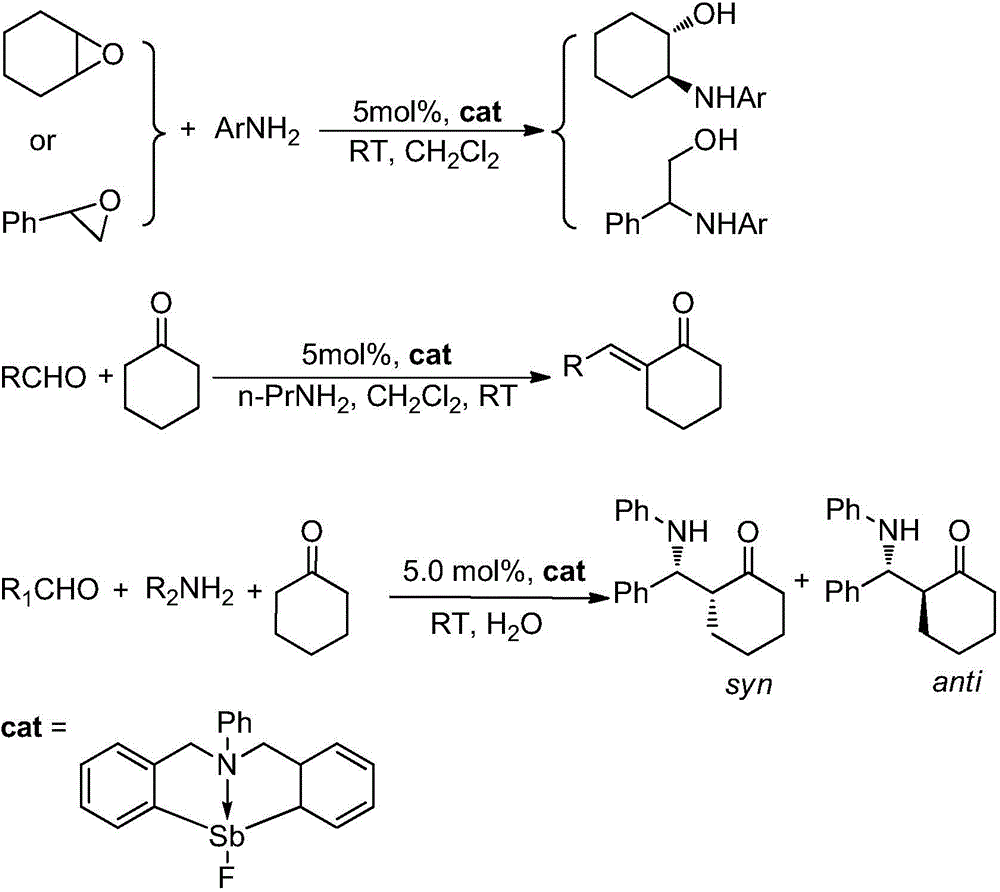
Catalytic synthesis application based on frustrated Lewis acid-base pair
InactiveCN104529796ASimple reaction conditionsEasy to operateOrganic compound preparationCarbonyl compound preparationSynthesis methodsStructural formula
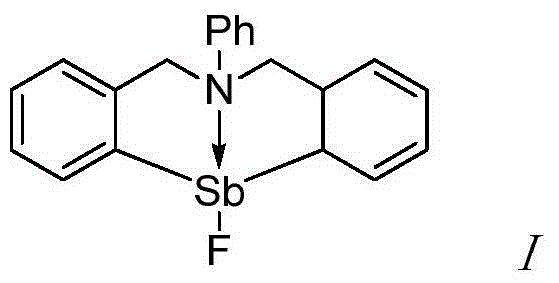
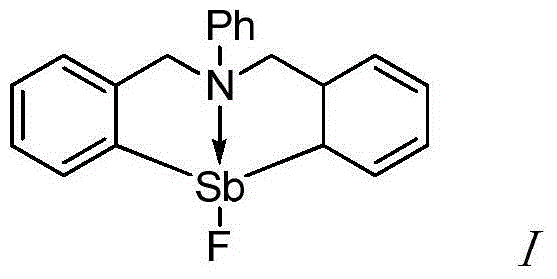
The invention provides a synthetic method for catalyzing beta-amino alcohol, alpha, beta-unsaturated ketone and beta-aminoketone based on a frustrated Lewis acid-base pair. A novel frustrated Lewis acid-base pair I which is stable in air is adopted as a catalyst and has the structural formula of PhN(CH2C6H4)2SbF, wherein Sb atom is bonded with two carbon atoms in a ligand and also forms a coordination bond together with nitrogen atom in the ligand. The method provides an efficient and environmental-friendly way for preparing a beta-amino alcohol compound from an epoxy compound and amine, preparing an alpha, beta-unsaturated ketone compound from aldehyde and ketone and preparing beta-aminoketone from aldehyde, ketone and amine. The frustrated Lewis acid-base pair is good in catalytic performance and can be repeatedly used, both the selectivity and the productivity of target products are close to 100%, the reaction conditions are simple, the operation is easy, the cost is low and the preparation process is environmental friendly.
Owner:HUNAN UNIV
Rapid UV (Ultraviolet) photo-cured ink and application method thereof
The invention relates to rapid UV (Ultraviolet) photo-cured ink and an application method thereof. The DI special ink is prepared from a composite photoinitiator, wherein the composite photoinitiatorconsists of di-2,6-difluoro-3-pyrrole phenyl titanocene dichloride, a ketoxime ester type photoinitiator, a thioxanthone photoinitiator, an acyl phosphine type photoinitiator, an alpha-hydroxyalkyl phenyl ketone photoinitiator and a morpholinyl-containing alpha-aminoketone photoinitiator; the di-2,6-difluoro-3-pyrrole phenyl titanocene dichloride accounts for 5-8% by weight of the total weight ofthe composite photoinitiator; the sum of weights of the other photoinitiators accounts for 90-95% by mass of the total weight of the composite photoinitiator. By adopting the composite photoinitiatorprovided by the invention, the problem that ink cannot be directly cured with ultraviolet light efficiently is solved; because of the low efficiency that ink is cured with ultraviolet light, a very long time for curing common ink is needed, although the problem of precise photo-curing is solved, low-efficiency photo-curing can be still not adopted in the industry.
Owner:深圳市容大感光科技股份有限公司 +1
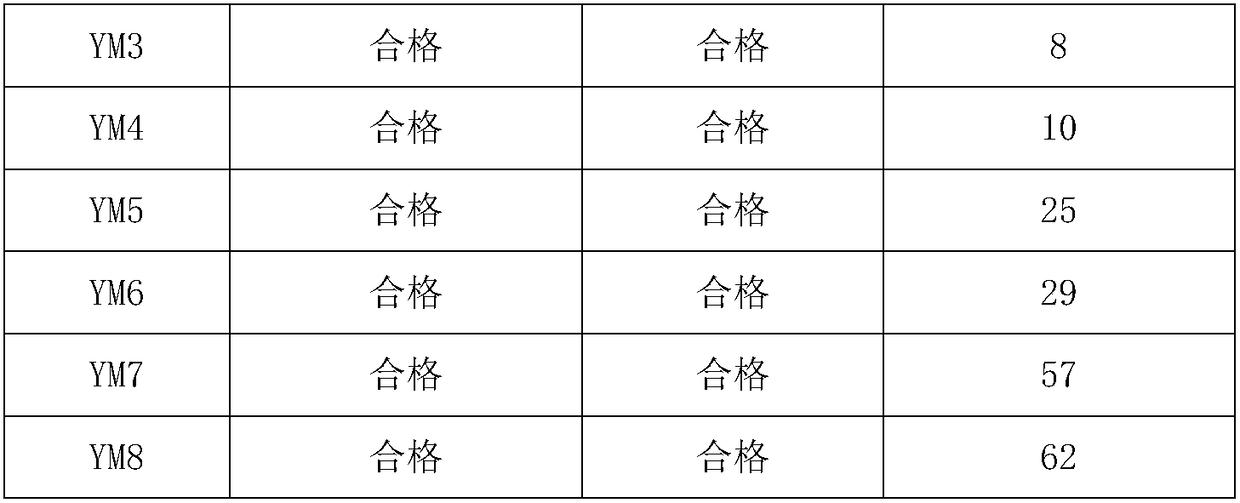
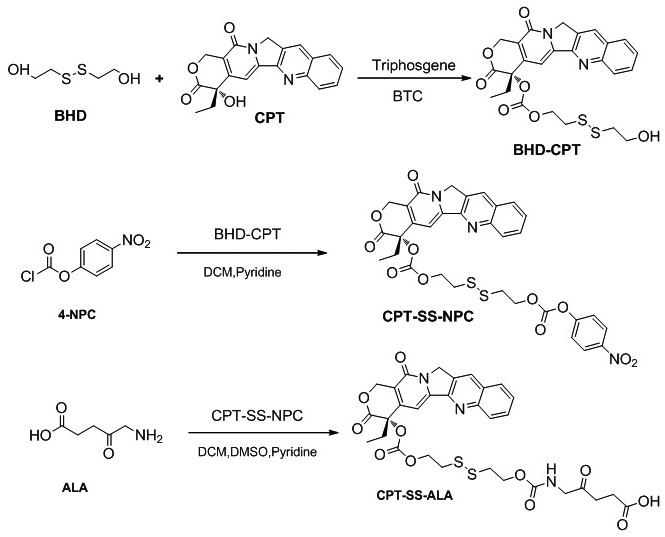
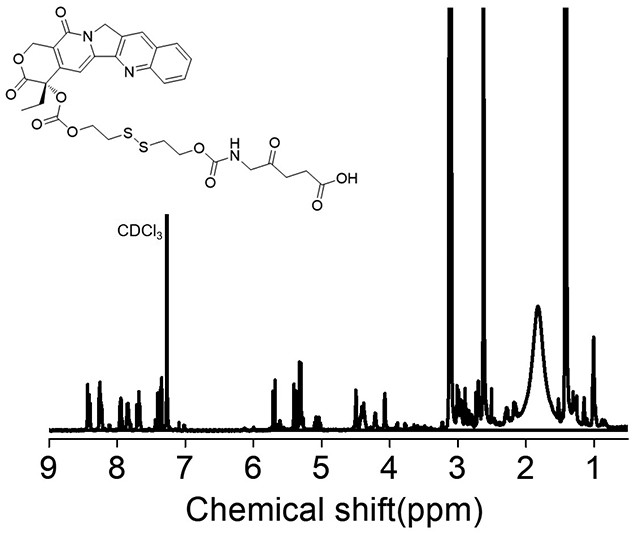
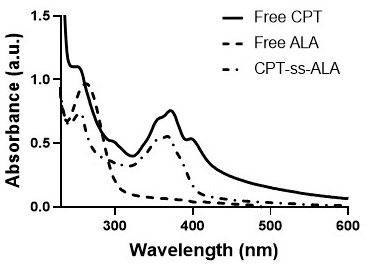
Preparation and application of 5-aminolevulinic acid-camptothecin micromolecular prodrug
InactiveCN111840574AIncrease upload volumeHigh selectivityOrganic active ingredientsEnergy modified materialsDisulfide bondingChemo therapy
The invention discloses preparation and application of a 5-aminolevulinic acid-camptothecin micromolecular prodrug. A preparation method comprises the following steps: (1) preparing a disulfide bond-containing camptothecin monomer BHD-CPT; (2) preparing CPT-SS-NPC; and (3) reacting a photosensitizer ALA with CPT-SS-NPC to prepare CPT-SS-ALA. Benefited from the amphiphilic structure of the drug conjugate, the drug conjugate can be self-assembled in water to form nanoparticles, and has the advantages of high micelle stability, controllable micelle shape, high drug loading capacity, low toxic andside effects, good drug controlled release and the like. In a reductive tumor microenvironment, disulfide bonds in the drug nanoparticles are broken, a chemotherapeutic drug CPT is released, meanwhile, the photosensitizer ALA continues to generate singlet oxygen under laser irradiation to kill cancer cells, and multi-modal synergistic treatment of tumors is achieved.
Owner:SOUTHWEST UNIV
Escherichia coli recombinant bacteria capable of high-producing 2, 5-dimethylpyrazine and construction method of escherichia coli recombinant bacteria
InactiveCN111411067AImprove unbalanced shortcomingsProlonged metabolic pathwayBacteriaMicroorganism based processesEscherichia coliHeterologous
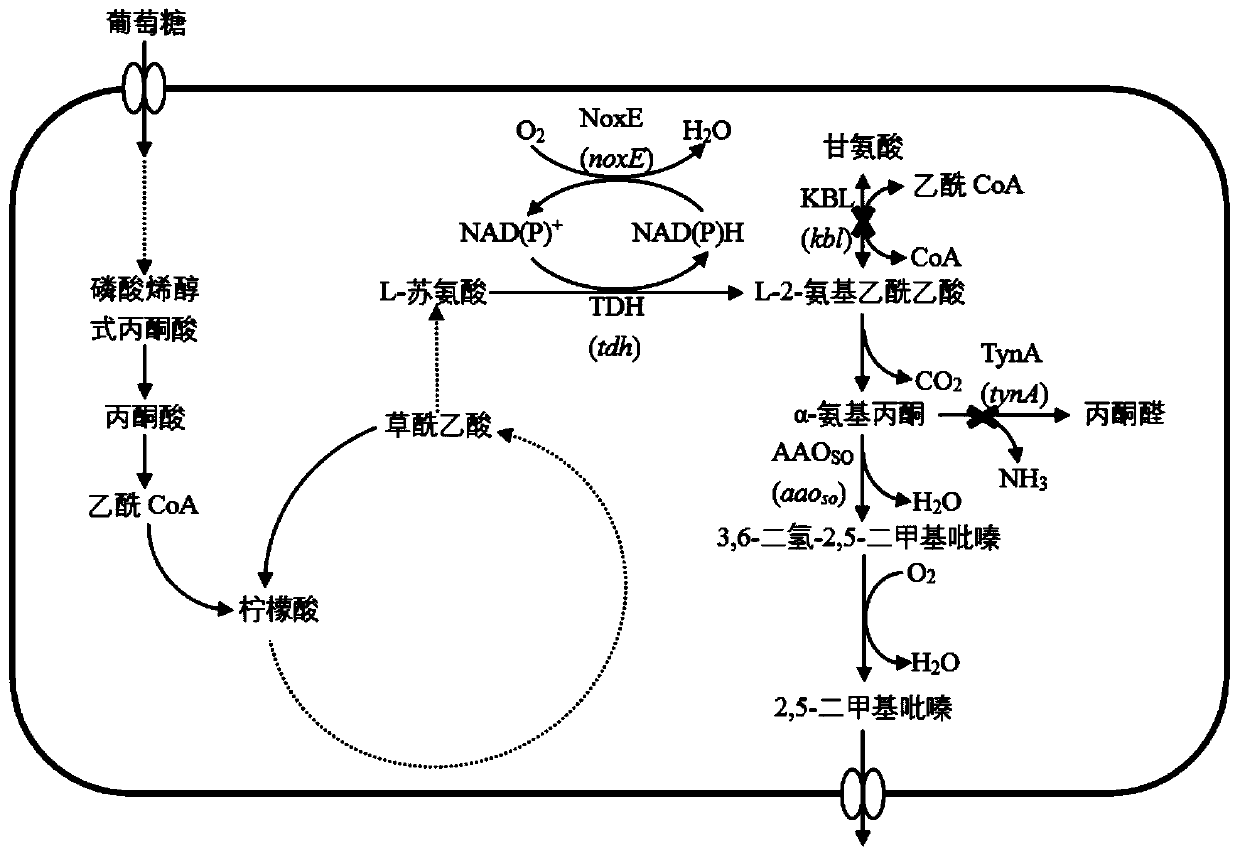
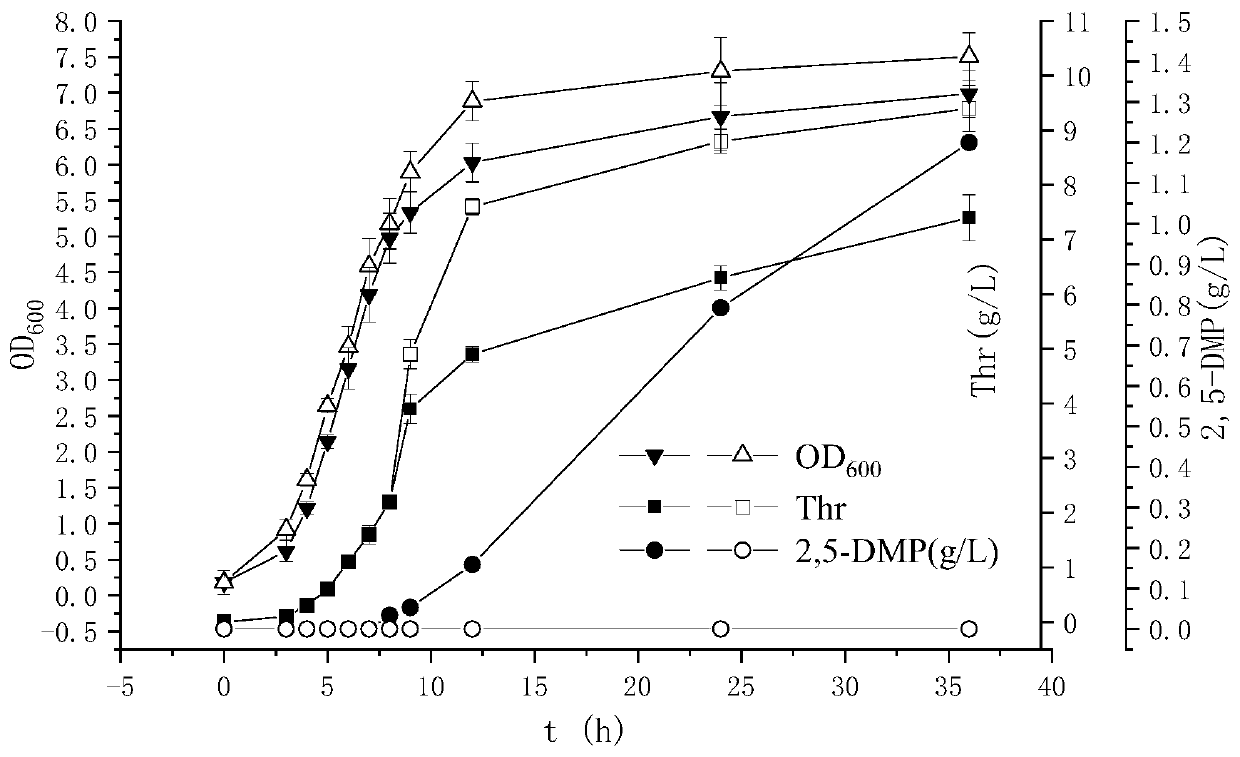
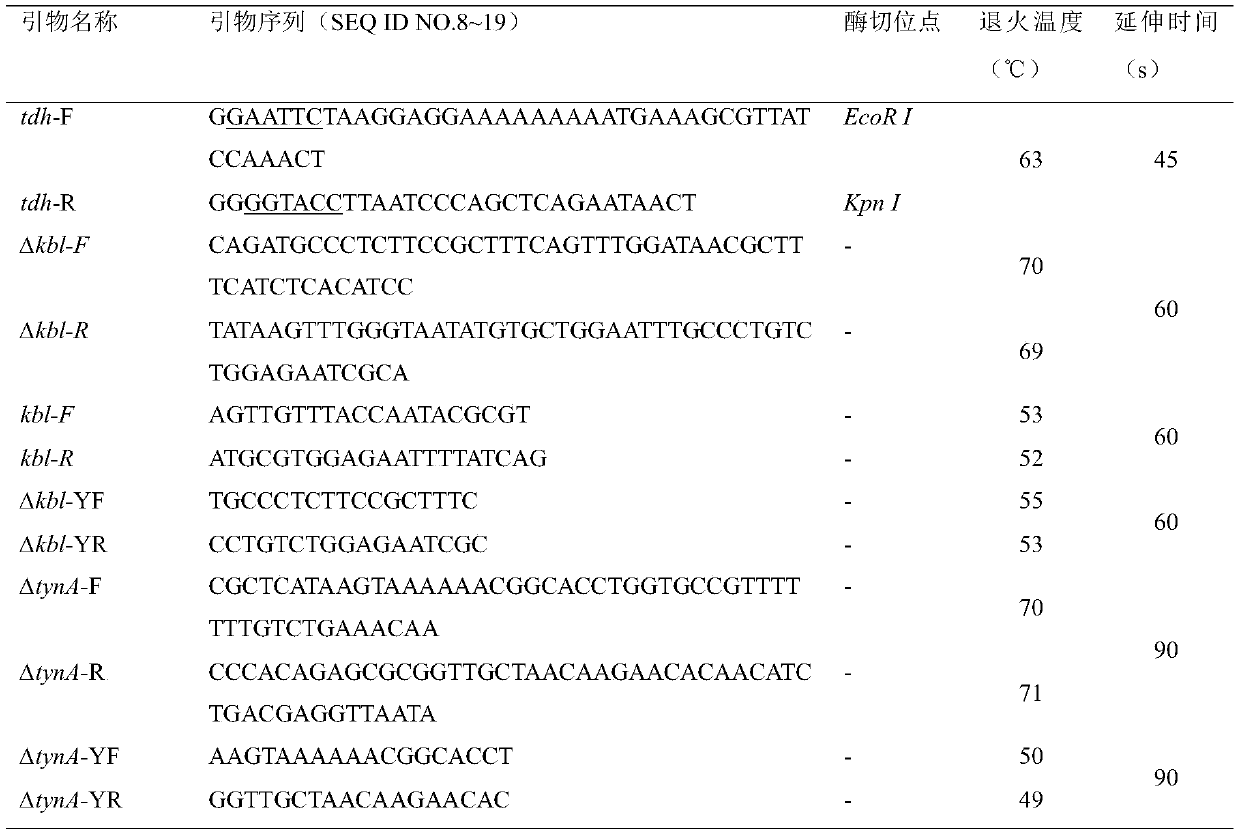
The invention discloses escherichia coli recombinant bacteria capable of high-producing 2, 5-dimethylpyrazine and a construction method of the escherichia coli recombinant bacteria, and belongs to thetechnical field of gene engineering. According to the escherichia coli recombinant bacteria and the construction method thereof, a genetic engineering method is applied, L-threonine dehydrogenase TDHis overexpressed in escherichia coli K-12 capable of high-producing L-threonine, and NADH oxidase NoxE derived from lactococcus microorganisms and aminoacetone oxidase AAOSO derived from streptococcus microorganisms are expressed in a heterologous manner, and meanwhile 2-amino-3-ketobutyric acid CoA ligase KBL and primary amine oxidase TynA are knocked out, so that a novel and efficient 2, 5-dimethylpyrazine synthetic route is constructed, and the problem of unbalance of cofactors in the recombinant bacteria is solved. By taking escherichia coli E. coli THR as an example, the accumulation amount of 2, 5-dimethylpyrazine reaches 1.2 + / -0.2 g / L through a 36h shaking flask fermentation experiment of the recombinant bacteria. According to the method, L-threonine high-producing strains are used as starting strains, the synthetic route of 2, 5-dimethylpyrazine in escherichia coli is successfully reconstructed, the defect of unbalanced intracellular cofactors is improved, and a new idea is provided for breeding 2, 5-dimethylpyrazine.
Owner:JIANGNAN UNIV
Process for producing optically active beta-amino alcohol
InactiveUS20050277791A1Readily availableEasy to produceOrganic compound preparationOrganic chemistry methodsGreek letter betaAlcohol
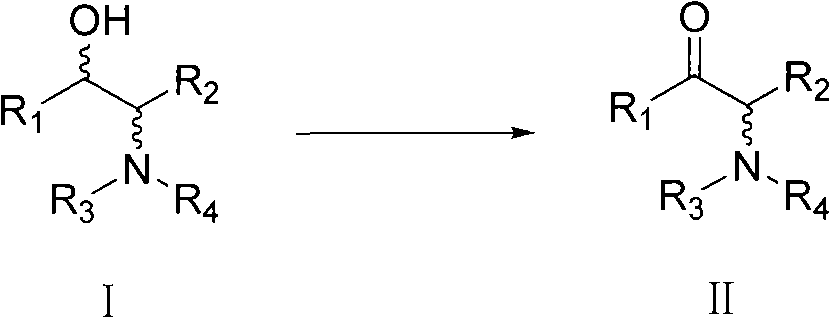
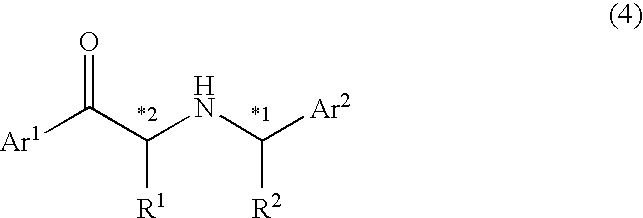
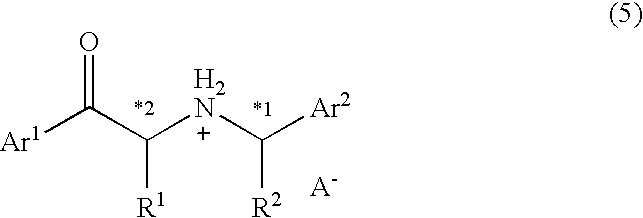
A process for easily producing an optically active β-amino alcohol useful as a pharmaceutical intermediate from an inexpensive, readily available starting material is provided. A readily available α-substituted ketone is reacted with an optically active amine to yield a diastereomer mixture of an optically active α-substituted aminoketone. One of the diastereomers is isolated optionally after the diastereomers are converted to salts with an acid. The optically active α-substituted aminoketone or a salt thereof thus isolated was stereoselectively reduced to yield an optically active β-substituted amino alcohol. The optically active β-substituted amino alcohol is subjected to hydrogenolysis to produce an optically active β-amino alcohol or a salt thereof.
Owner:KANEKA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com