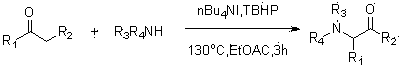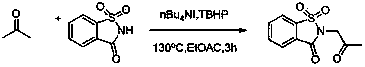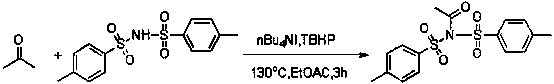Method for synthesizing alpha-amino ketone from ketone and imine
An amino ketone, imine technology, applied in the preparation of sulfonic acid amides, organic chemistry and other directions, to achieve the effect of simple experimental steps, a wide range of substrates, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add ethyl acetate as an organic solvent (3.0 ml) into the pressure-resistant tube, add 1 times the amount of saccharin to fully dissolve (54.9 mg, 0.30 mmol), and then add 5 times the amount of acetone (0.111 liters, 1.50 mmol), 0.20 times the amount of catalyst tetrabutylammonium iodide (22.20 mg, 0.06 mmol), 2 times the amount of oxidant tert-butanol peroxide (0.109 ml, 0.60 mmol), and seal the pressure-resistant tube , heating at 130 o C, stirred for 3 hours (the above-mentioned dosage ratios are molar ratios, and can be scaled up in proportion); after the reaction, the system was cooled to room temperature, and then passed through methylene chloride (CH 2 Cl 2 ) extraction (15ml×3 times); the obtained organic phase was removed on a rotary evaporator to remove the organic solvent; the obtained solid was separated and purified by column chromatography to obtain a white solid with a yield of 91%. The reaction can be expressed by the following equation:
[0023]
Embodiment 2
[0025] Add ethyl acetate as an organic solvent (3.0 ml) into the pressure-resistant tube, add 1 times the amount of saccharin to fully dissolve (54.9 mg, 0.3 mmol), and then add 5 times the amount of acetone (0.111 liters, 1.5 mmol), 0.15 times the amount of catalyst tetrabutylammonium iodide (16.61 mg, 0.045 mmol), 2 times the amount of oxidant tert-butanol peroxide (0.109 ml, 0.6 mmol), and seal the pressure-resistant tube , heating at 130 o C, stirred for 3 hours (the above-mentioned dosage ratios are molar ratios, and can be scaled up in proportion); after the reaction, the system was cooled to room temperature, and then passed through methylene chloride (CH 2 Cl 2 ) extraction (15ml×3 times); the obtained organic phase was removed on a rotary evaporator to remove the organic solvent; the obtained solid was separated and purified by column chromatography to obtain a white solid with a yield of 89%. The reaction can be expressed by the following equation:
[0026]
Embodiment 3
[0028] Add ethyl acetate as an organic solvent (3.0 ml) into the pressure-resistant tube, add 1 times the amount of saccharin to fully dissolve (54.9 mg, 0.30 mmol), and then add 5 times the amount of acetone (0.111 liters, 1.50 mmol), 0.20 times the amount of catalyst tetrabutylammonium iodide (22.20 mg, 0.06 mmol), 1.5 times the amount of oxidant tert-butanol peroxide (0.081 ml, 0.45 mmol), and seal the pressure-resistant tube , heating at 130 o C, stirred for 3 hours (the above-mentioned dosage ratios are molar ratios, and can be scaled up in proportion); after the reaction, the system was cooled to room temperature, and then passed through methylene chloride (CH 2 Cl 2 ) extraction (15ml×3 times); the obtained organic phase was removed on a rotary evaporator to remove the organic solvent; the obtained solid was separated and purified by column chromatography to obtain a white solid with a yield of 85%. The reaction can be expressed by the following equation:
[0029] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com