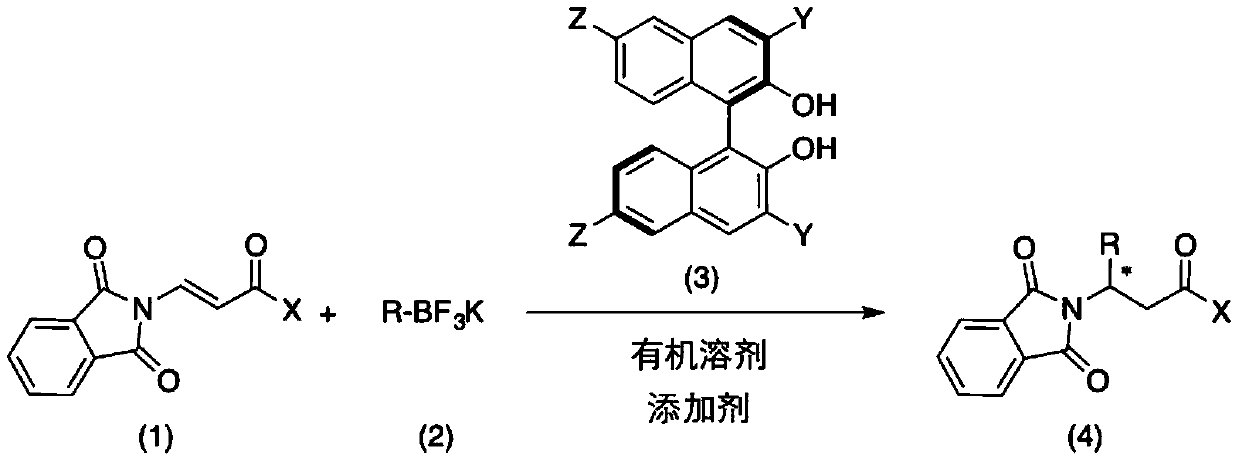
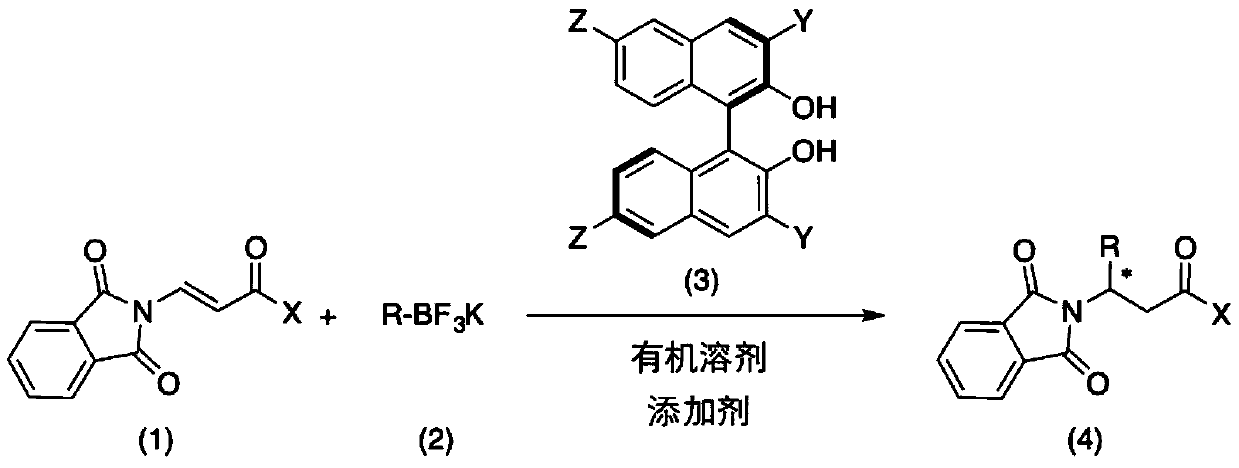
Method for catalytically asymmetrically synthesizing chiral beta-aminoketone derivative
An aminoketone and asymmetric technology, which is applied in the field of catalytic asymmetric synthesis of chiral β-aminoketone derivatives, can solve the problems of narrow application range of substrates and nucleophiles, expensive metal catalysts, and high requirements for substrate structures. , achieve good application prospects and social value, reduce reaction cost, high yield and high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: (R)-3,3'-bis(2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl)-6,6'-bis(trifluoromethyl) -[1,1'-Binaphthyl]-2,4-2'-diol preparation
[0026] N 2 Under protection, sequentially add (R)-2,2'-bis(methoxymethyl ether)-6,6'-bis(trifluoromethyl)-1,1'-bibi in a 100mL two-necked bottle Naphthalene (2.96 mmol, 1.51 g) and THF (24 mL). The temperature of the system was cooled to 0°C, a 2.5M n-butyllithium n-hexane solution (8.88 mmol, 3.6 mL) was added, and the mixture was stirred at this temperature for 2 hours. The temperature of the system was then cooled to -78°C, and perfluorotoluene (20.72 mmol, 2.9 mL) was added dropwise. After the dropping, the system was slowly raised to room temperature and stirred at room temperature for 12 hours. After the reaction was completed, the reaction was quenched with saturated aqueous ammonium chloride solution (10 mL) in an ice bath, and then extracted three times with ether (3×10 mL). The combined extract phase was washed with brin...
Embodiment 2
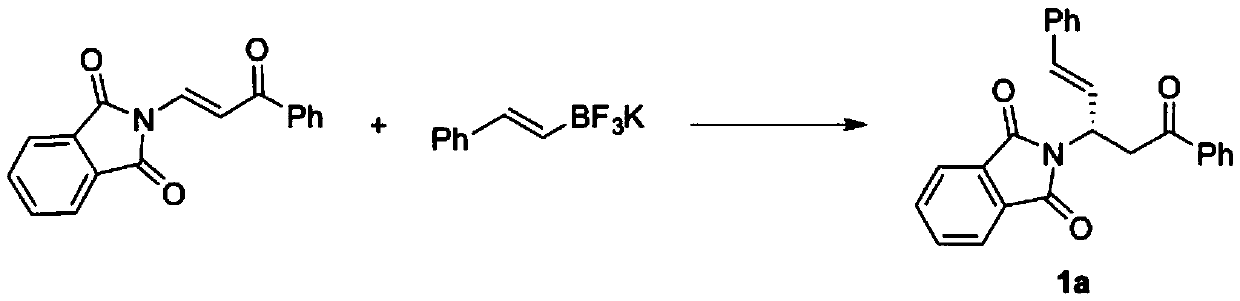
[0027] Example 2: (S,E)-2-(5-oxo-1,5-diphenylpentene-1-en-3-yl)isoindole-1,3-dione (1a) preparation
[0028] N 2 Under protection, add additives, β-phthalimide propylene ketone substrate (0.1 mmol, 1.0 equiv), and chiral binaphthol catalyst (L3 or L6) (0.05 to 0.5 equiv) in a 25 mL reaction flask. ), potassium styryl trifluoroborate (1 to 5 equiv) and anhydrous trifluorotoluene (1 mL), the reaction system is sealed and heated (reaction temperature 25 to 90°C). The reaction was monitored by TLC analysis until the reaction was completed, the reaction system was filtered through celite and concentrated, and the concentrated crude product obtained was separated and purified by column chromatography to obtain the target compound 1a.
[0029] The characterization data of the target compound 1a are as follows:
[0030] White solid, [α] D 20 = +29.6(c1.0,CHCl 3 ). MP: 100.0-103.9°C. 1 H NMR(600MHz, CDCl 3 )δ7.96(d,J=7.8Hz,2H), 7.83(dd,J=5.1,3Hz,2H), 7.69(dd,J=5.4,3Hz,2H), 7.55(t,J=7.8Hz, ...
Embodiment 3
[0037] Example 3: Binaphthol-catalyzed reaction method expansion of β-phthalimide acrylone and aromatic substituted potassium trifluoroborate
[0038] N 2 Under protection, add additives, β-phthalimide propylene ketone (0.1 mmol, 1.0 equiv), chiral binaphthol catalyst L6 (0.01 mmol, 0.1 equiv), aryl or Heterocyclic aryl substituted styryl trifluoroborate potassium salt (0.2 mmol, 2 equiv) and anhydrous trifluorotoluene (2 mL). The reaction system was sealed and heated to 60°C. After monitoring the reaction by TLC until the reaction is complete, the reaction system is filtered through celite and concentrated, and the concentrated crude product is separated and purified by column chromatography to obtain the corresponding target compound 1a-1l. The reaction formula of the reaction process is as follows:
[0039]
[0040] In Example 3, the reaction conditions were changed as follows: the type and amount of additives, the type and reaction time of styryl trifluoroborate potassium salt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com