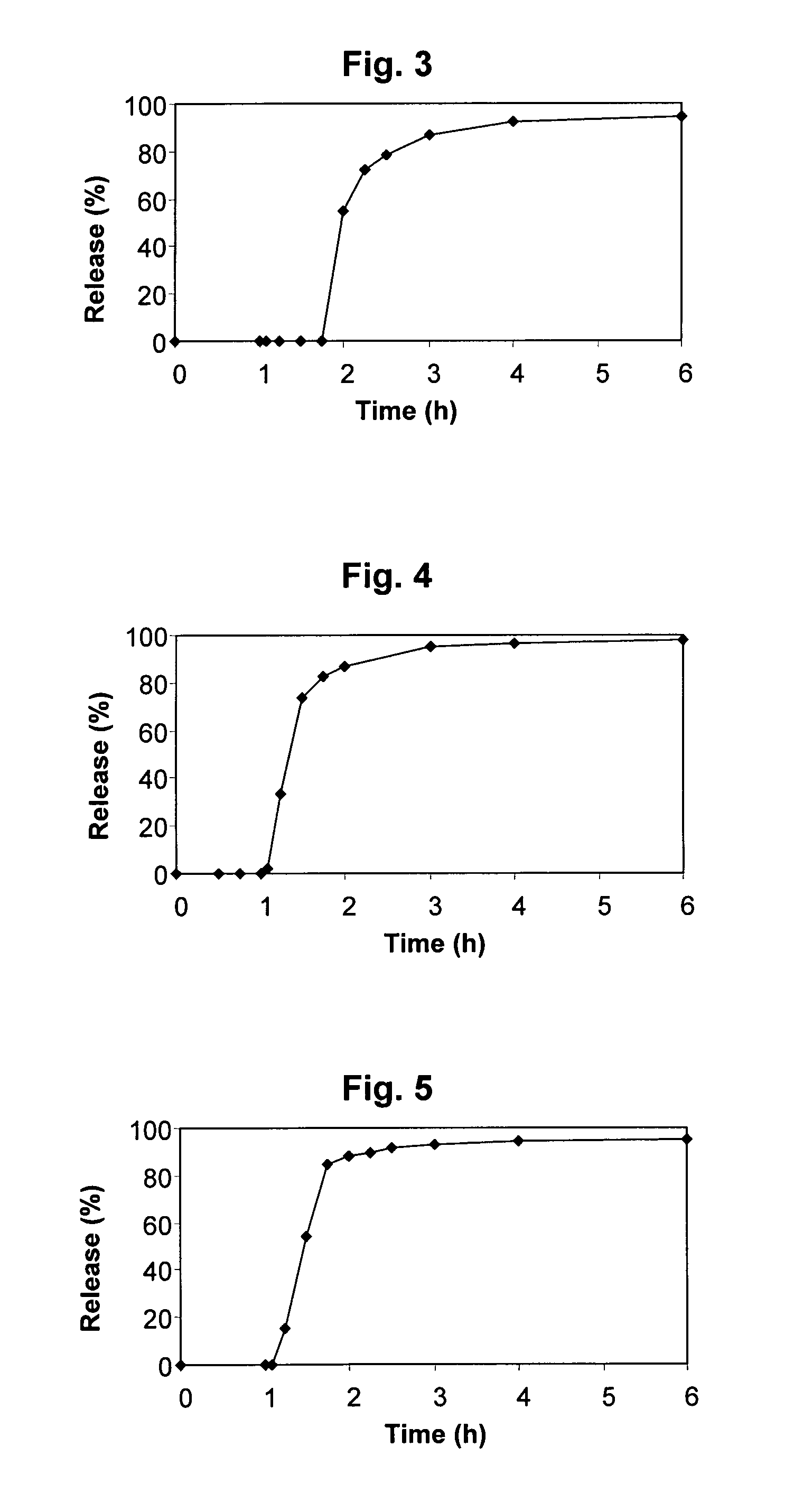
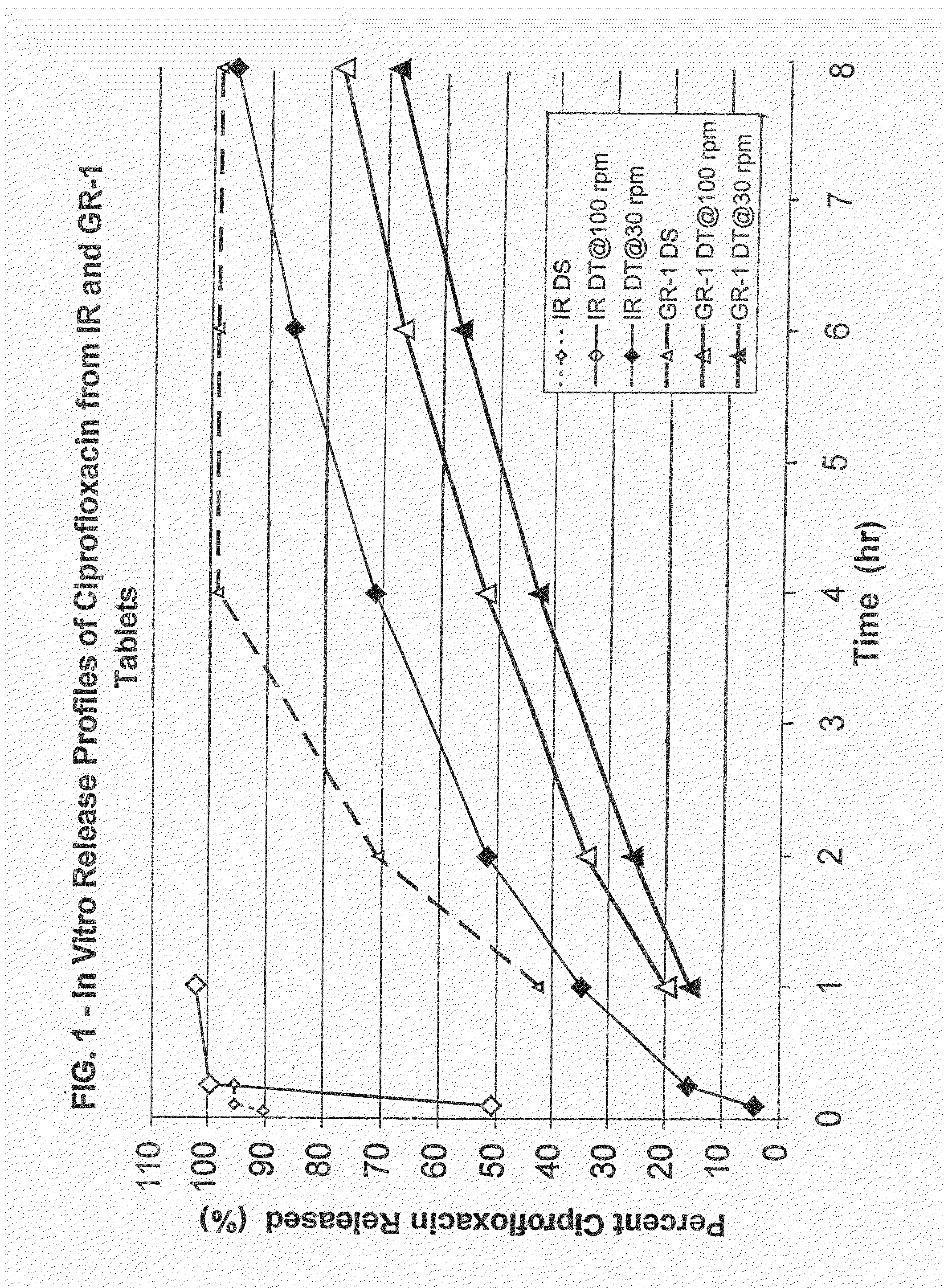
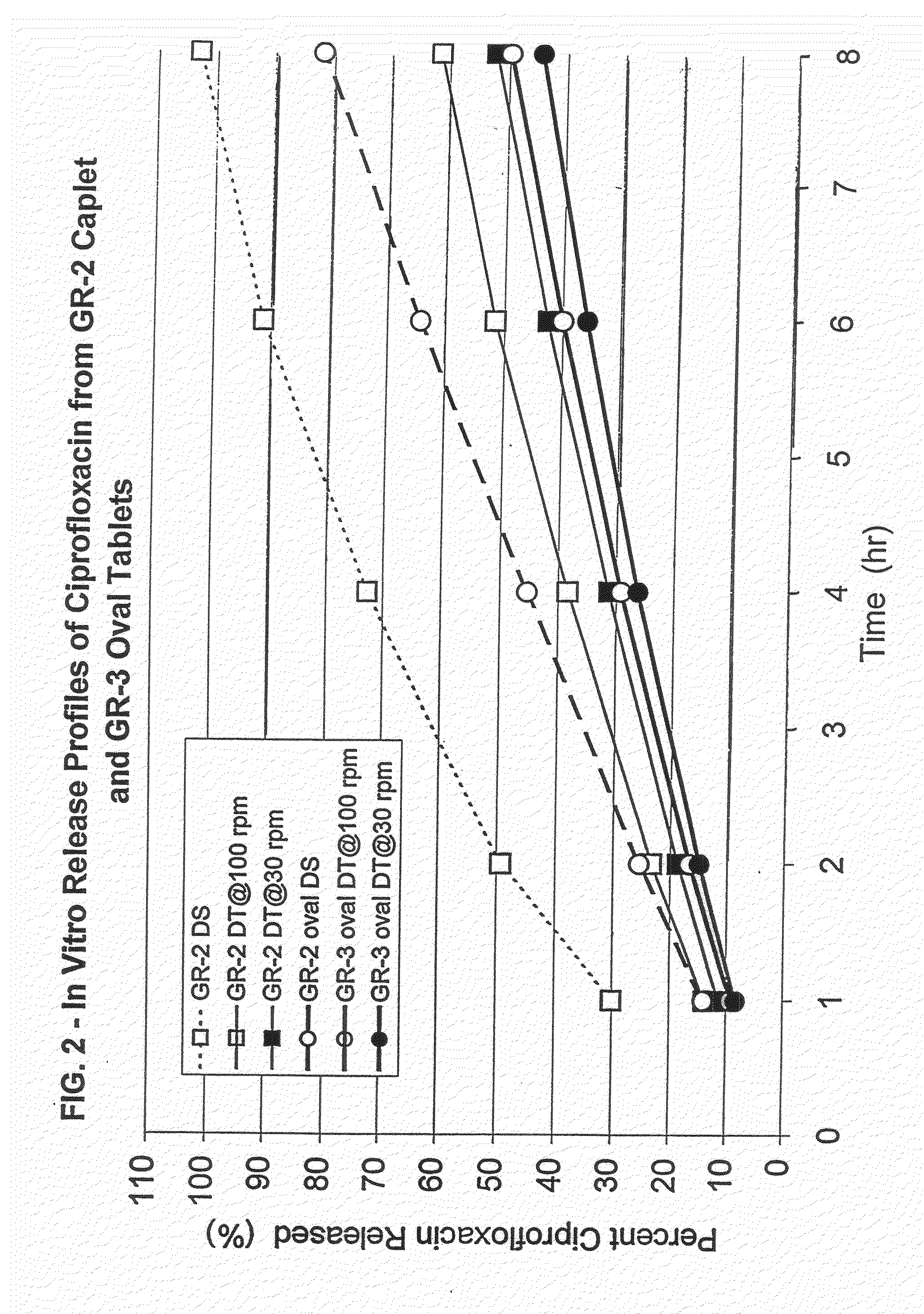
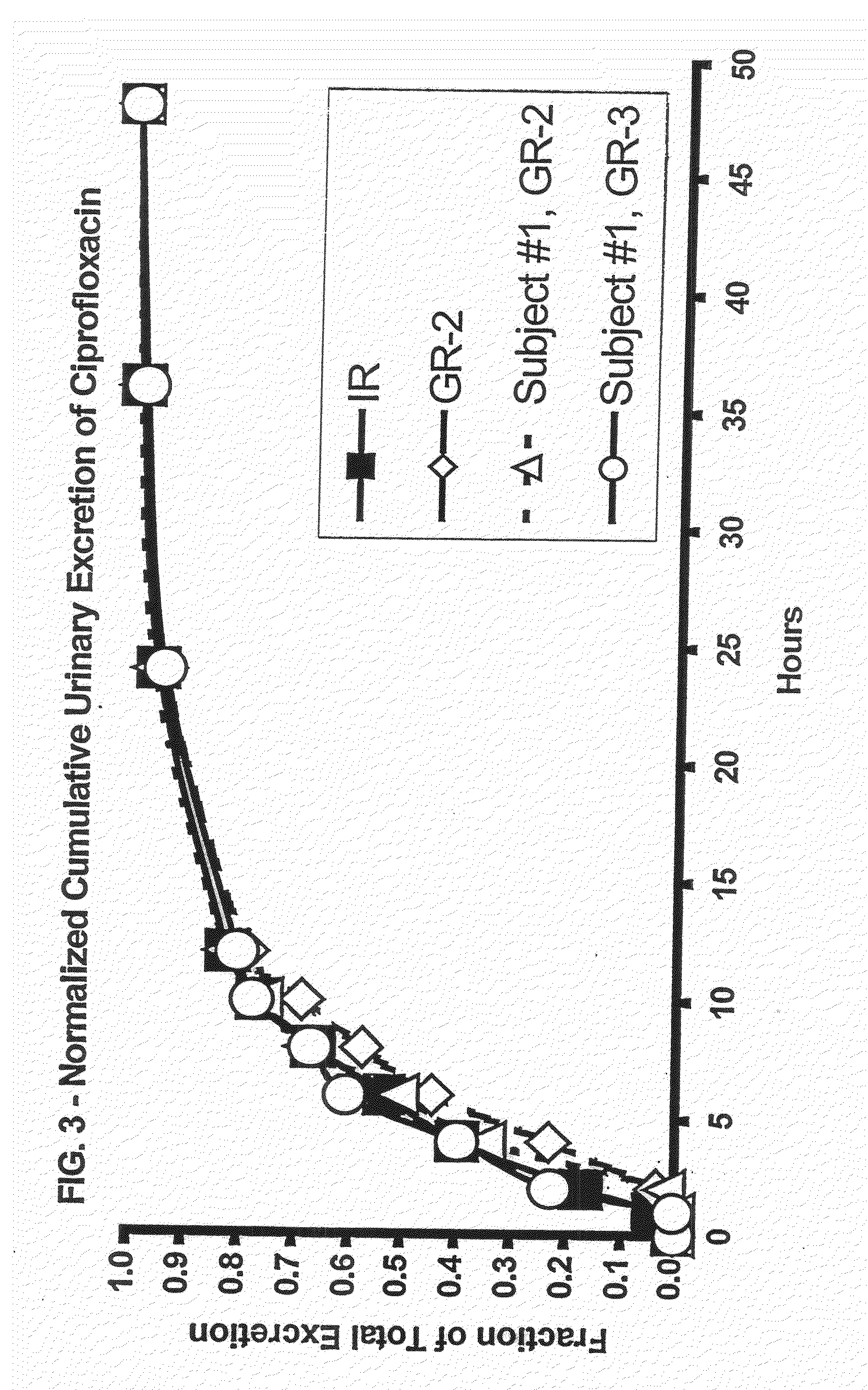
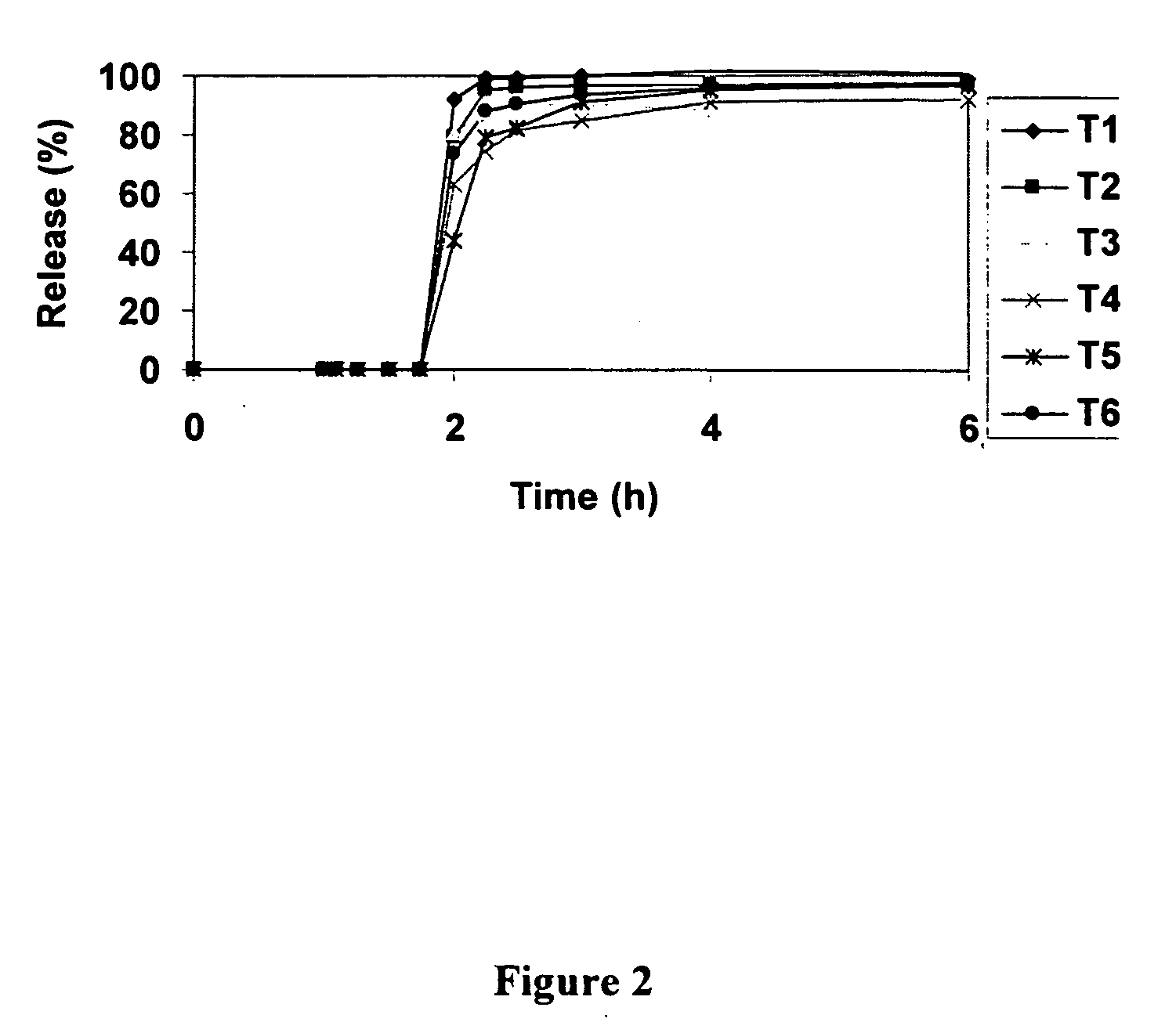
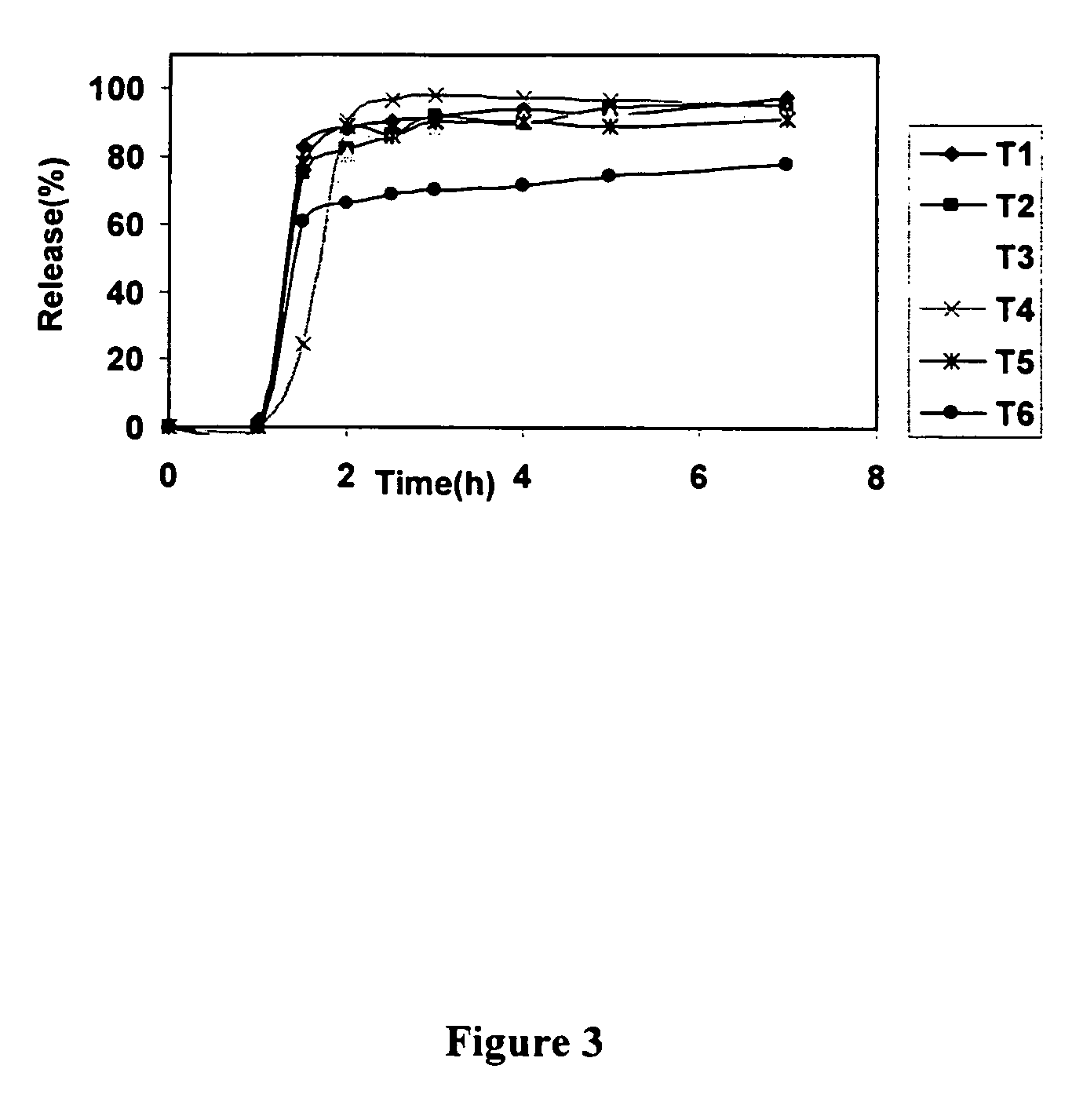
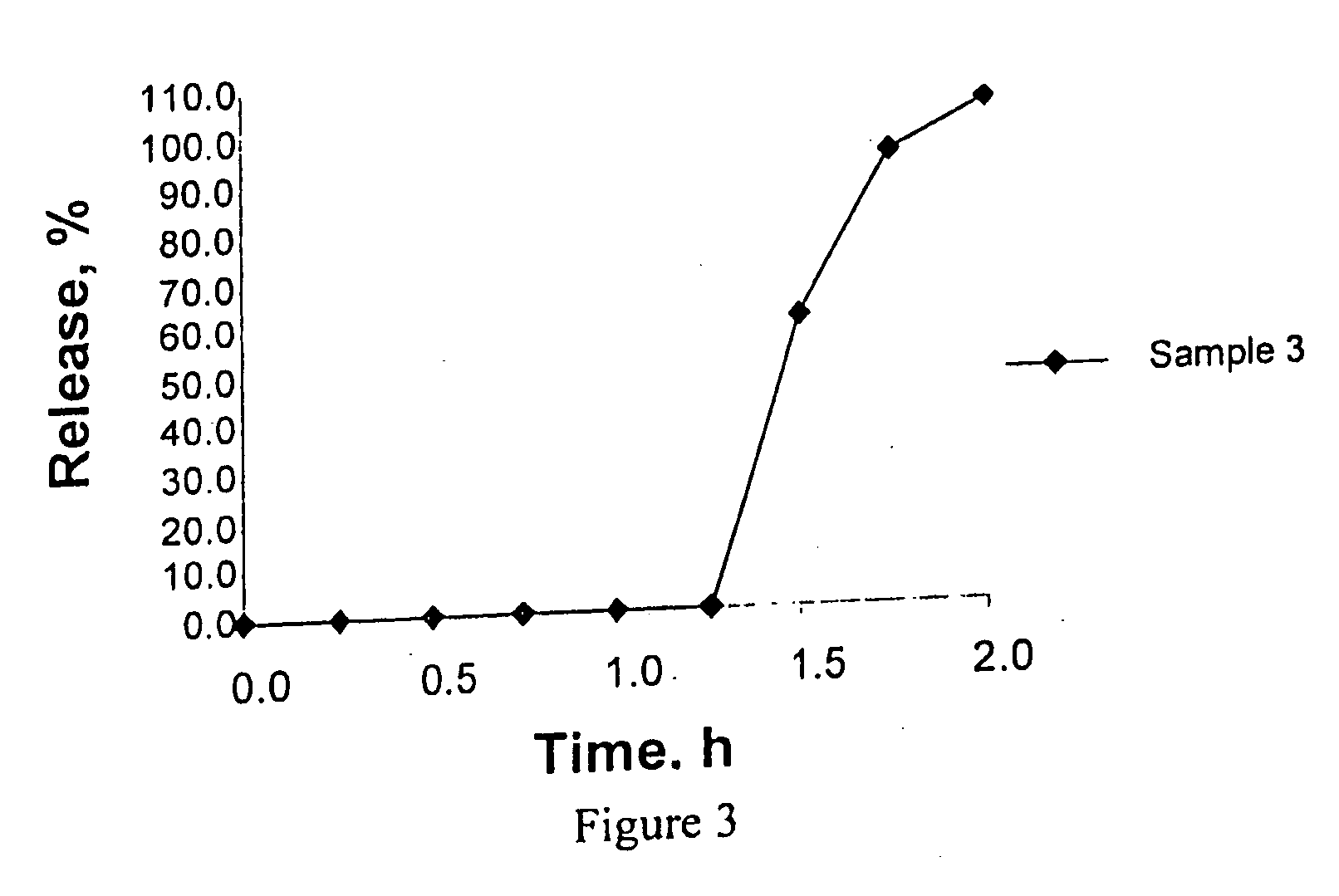
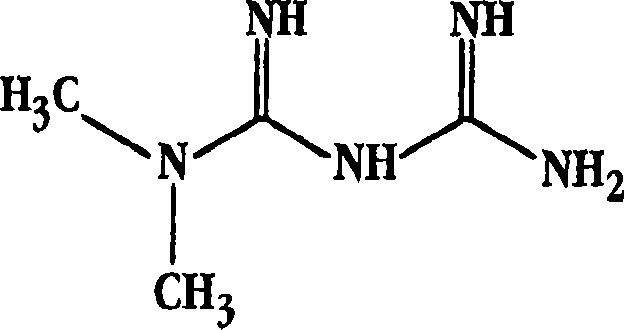
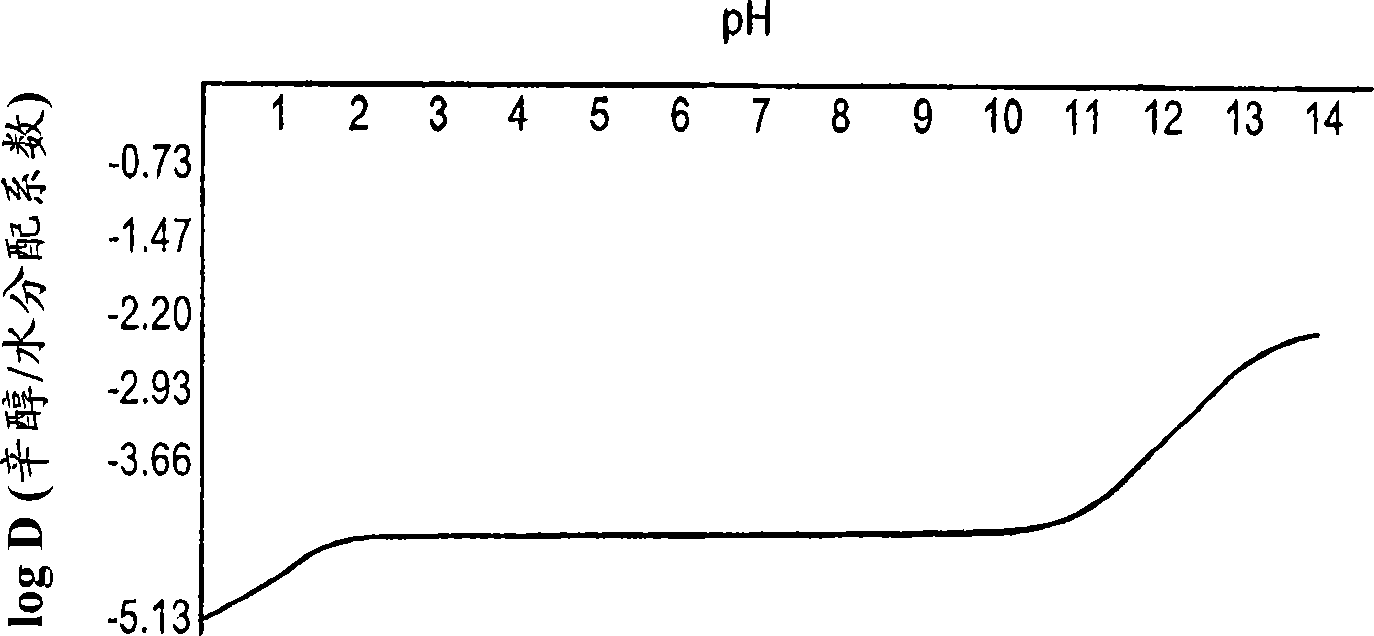
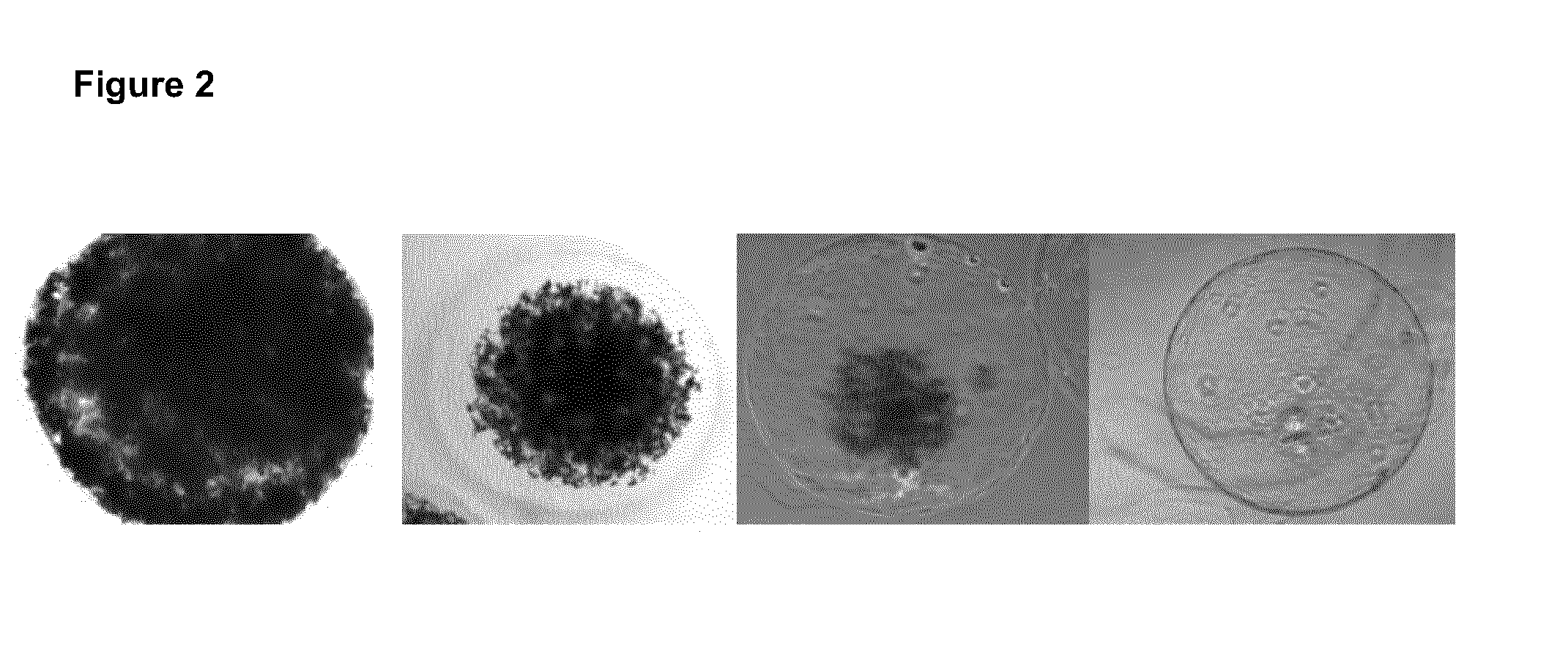Patents
Literature
65 results about "Lower Gastrointestinal Tract" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
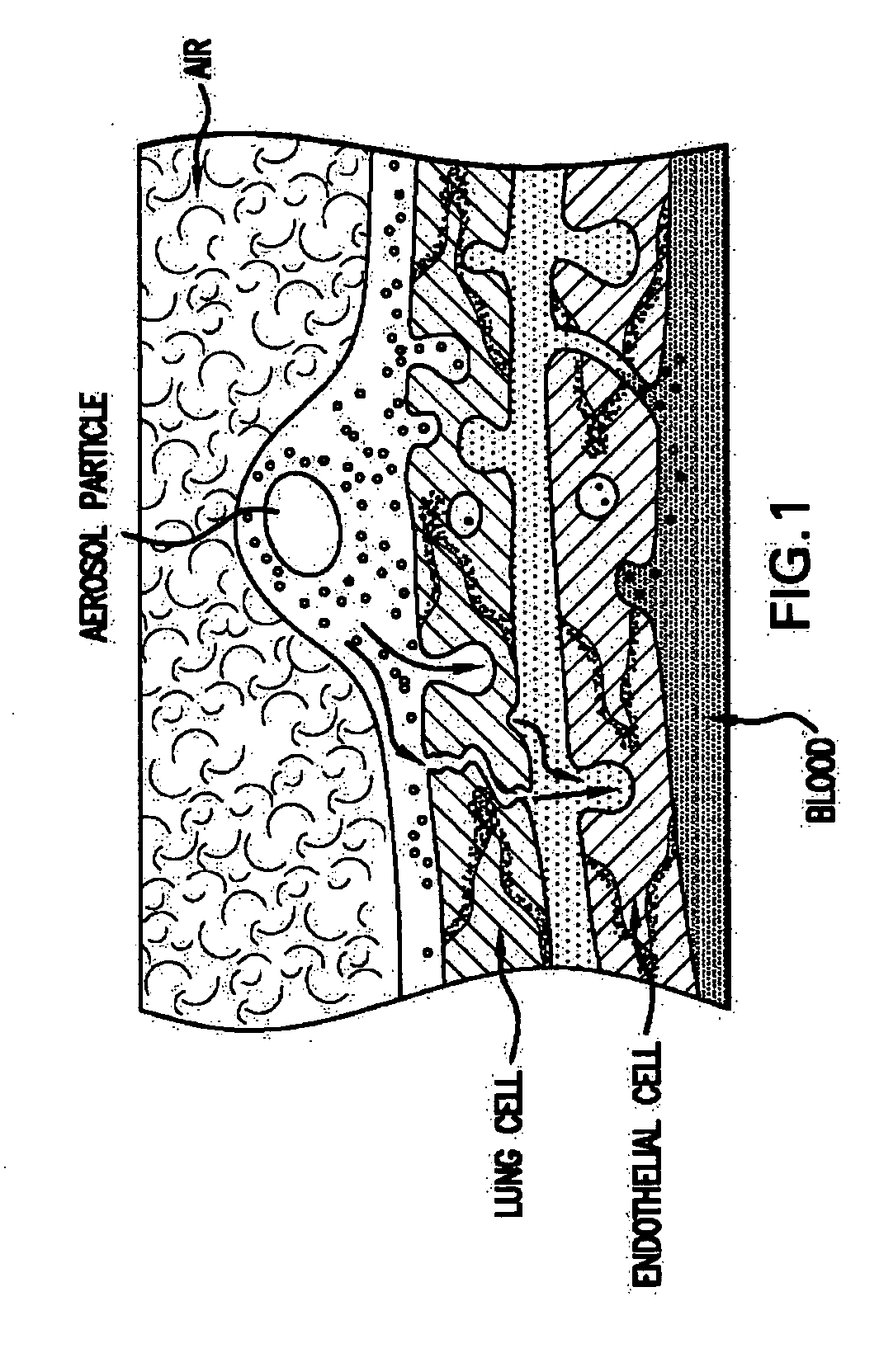
The gastrointestinal (GI), or digestive, tract extends from mouth to anus (see the image below). The division of the GI tract into upper and lower is a matter of some confusion and debate.
Colon-targeted oral formulations of cytidine analogs
InactiveUS20080057086A1Improve bioavailabilityBiocideCarbohydrate active ingredientsDiseaseLower Gastrointestinal Tract
The present invention provides an oral formulation of a cytidine analog, including, 5-azacytidine, for delivery to the lower gastrointestinal tract, including, the large intestine; methods to treat diseases associated with abnormal cell proliferation by treatment with the oral formulations of the present invention; and methods to increase the bioavailability of a cytidine analog upon administration to a patient by providing an oral formulation of the present invention.
Owner:PHARMION
Dosage forms of bisphosphonates
ActiveUS20050260262A1Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyUpper gastrointestinal
Oral dosage forms of a bisphosphonate comprised of a safe and effective amount of a pharmaceutical composition comprising a bisphosphonate, a chelating agent, and, means for effecting delayed release of the bisphosphonate and the chelating agent in the lower gastrointestinal tract provide delivery of the pharmaceutical composition to the lower gastrointestinal tract of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between bisphosphonates and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of the bisphosphonate and the chelating agent to the lower GI tract, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
Dosage forms of bisphosphonates
ActiveUS7645459B2Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyLower Gastrointestinal Tract
Oral dosage forms of a bisphosphonate comprised of a safe and effective amount of a pharmaceutical composition comprising a bisphosphonate, a chelating agent, and, means for effecting delayed release of the bisphosphonate and the chelating agent in the lower gastrointestinal tract provide delivery of the pharmaceutical composition to the lower gastrointestinal tract of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between bisphosphonates and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of the bisphosphonate and the chelating agent to the lower GI tract, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
Nutritional supplement and method of delivery
InactiveUS20020168429A1Easy to prepareEasily delivered orallyBiocideUnknown materialsBiotechnologyLower Gastrointestinal Tract
A method of delivering dietary supplements, in the form of intact anthocyanins, into the lower gastrointestinal tract of a body for absorption from an aqueous medium comprising: expressing juice from one or more fruits, which fruits include anthocyanins, thereby yielding a juice portion and a pomace portion; concentrating the juice portion to yield a juice concentrate; mixing the juice concentrate with the pomace portion; drying the juice-infused pomace to yield a free-flowing, non-hygroscopic powder formulation to yield the dietary supplement; and orally ingesting the dietary supplement in the form of capsules, tablets, shakes, drinks, energy supplements, energy bars, and the like.
Owner:MANN DOUGLAS G
Methods for improving gut health
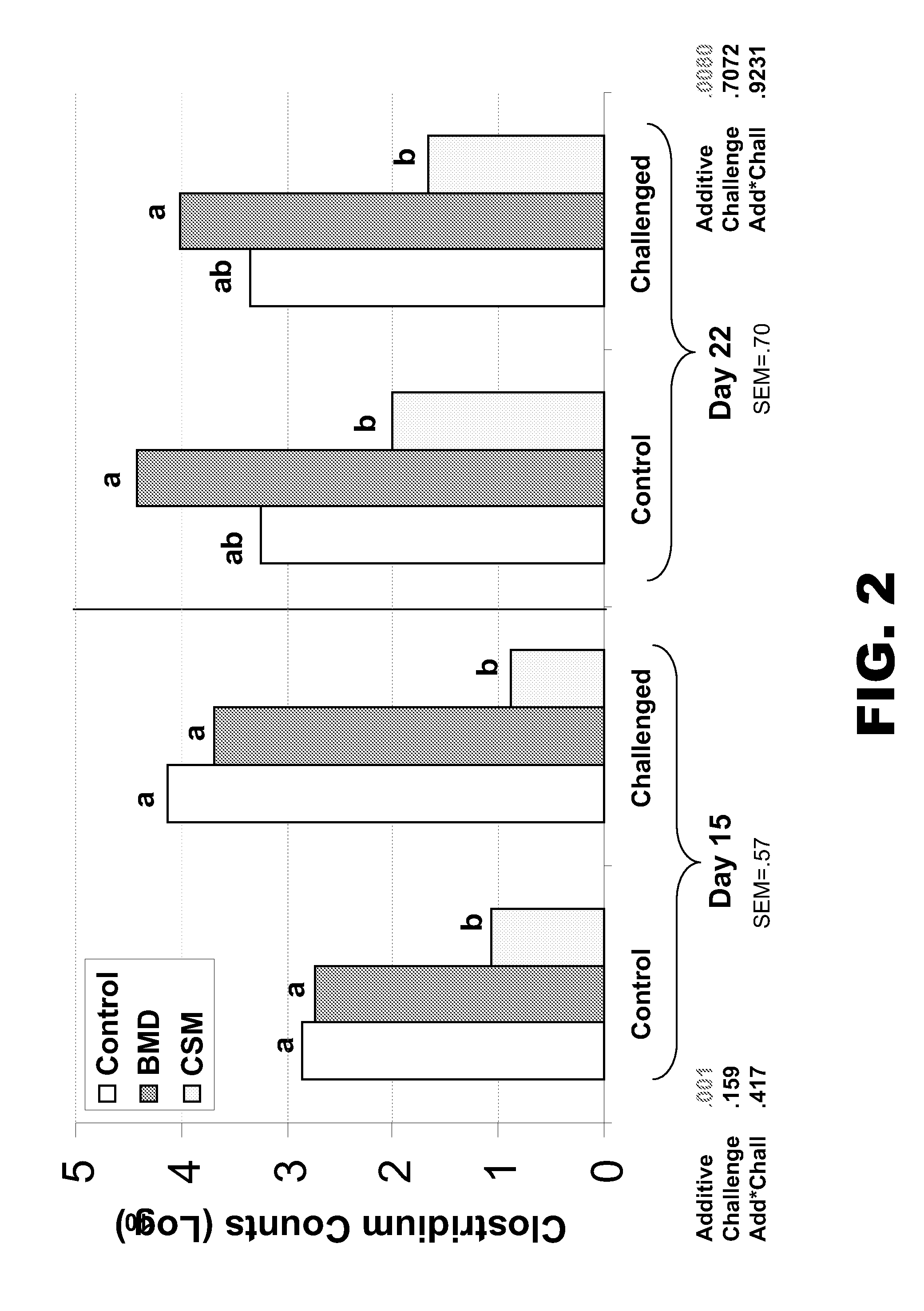
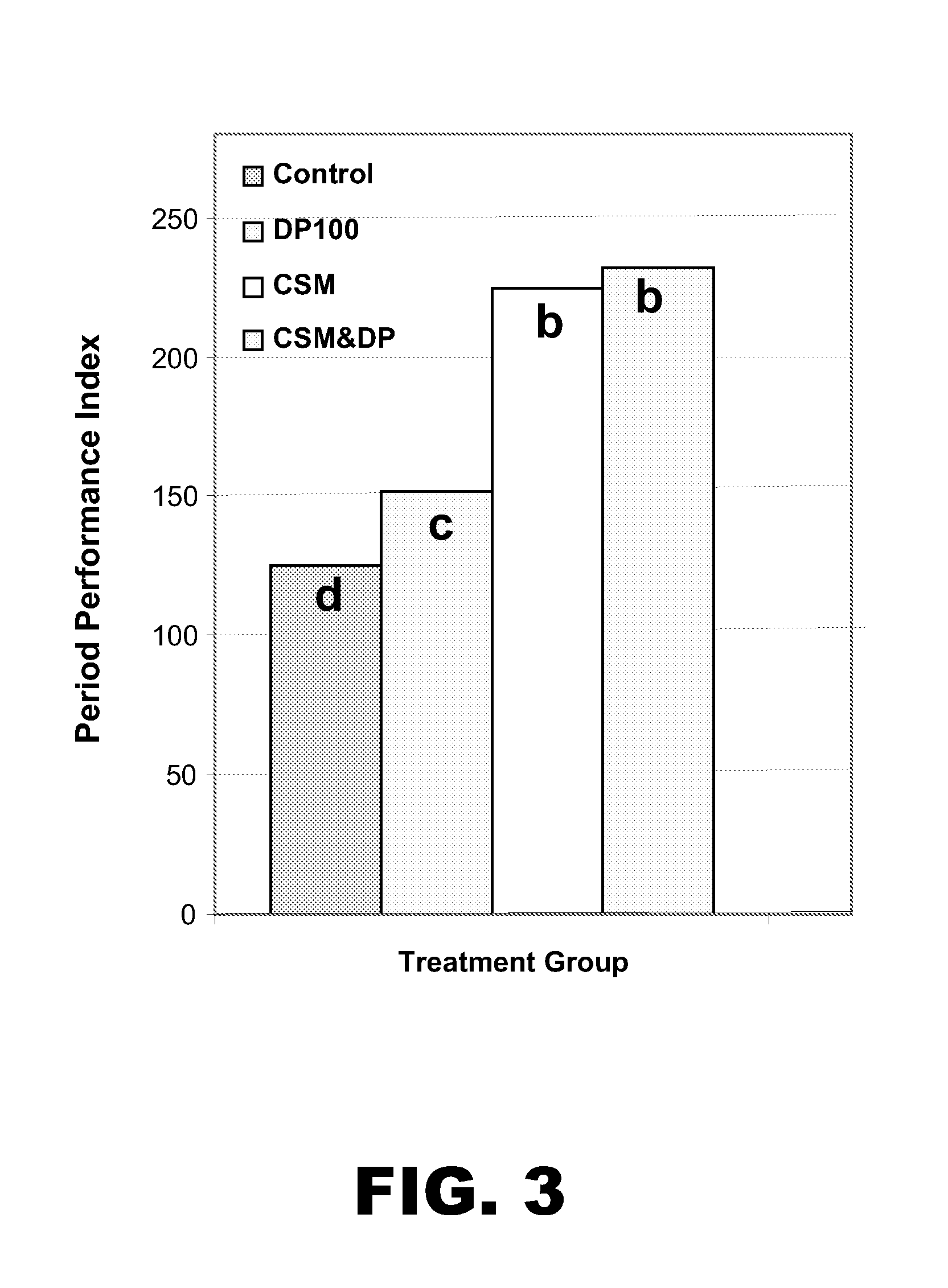
ActiveUS20120225050A1Reduce the populationReducing protein flowAntibacterial agentsPeptide/protein ingredientsLower Gastrointestinal TractDietary protein
The present invention provides methods for improving gut health. In particular, the invention provides methods for improving gut health by improving the digestibility of dietary proteins, decreasing the flow of protein to the lower gastrointestinal tract, and / or decreasing the levels of Clostridium bacteria the upper intestinal tract of a subject. The methods comprise administering to the subject a supplement consisting essentially of at least one protease.
Owner:NOVUS INTERNATIONAL INC
Compositions and dosage forms for enhanced absorption of iron
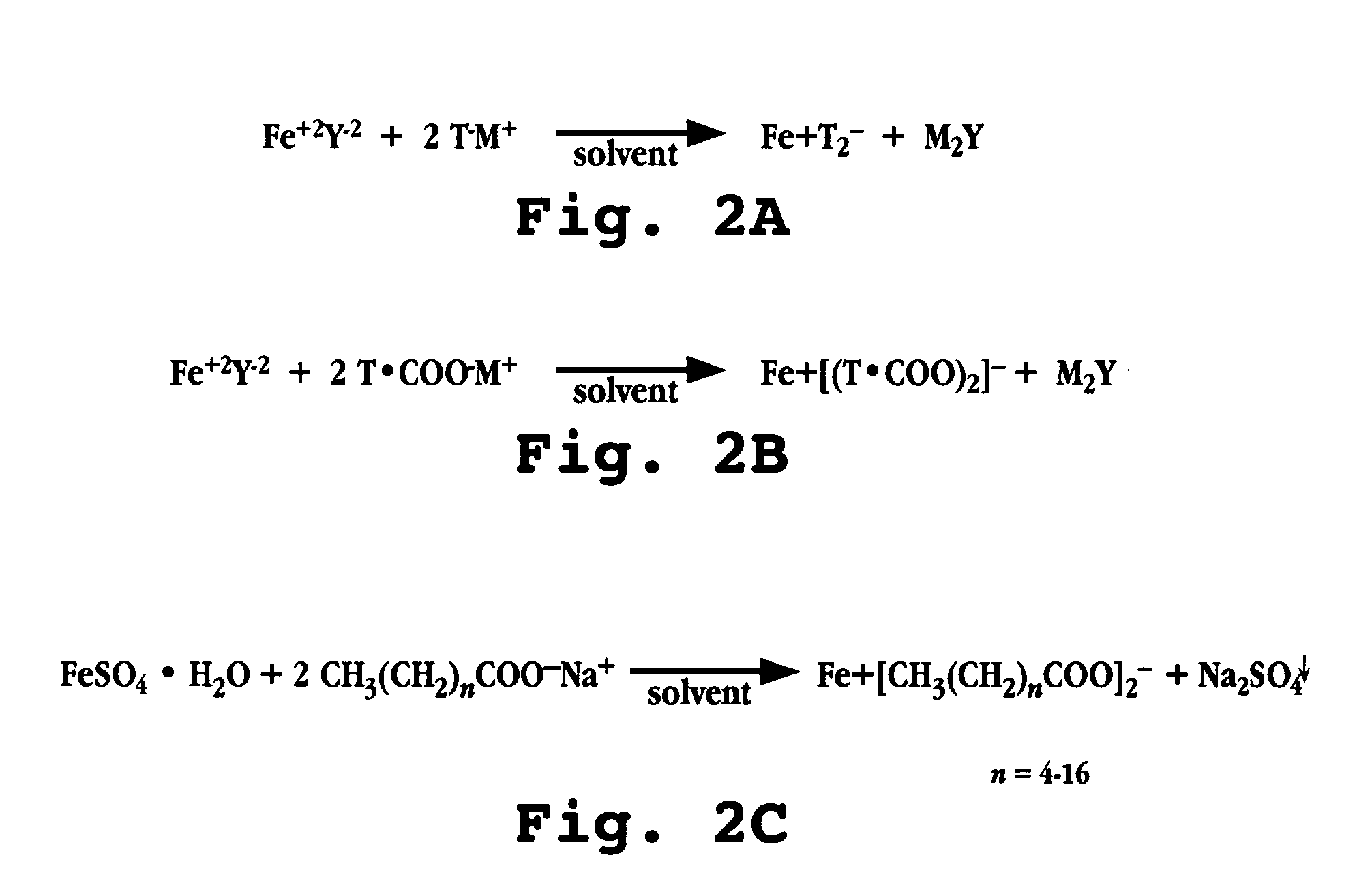
InactiveUS20050163849A1Heavy metal active ingredientsBiocideLower Gastrointestinal TractEnhanced absorption
A complex comprised of iron and a transport moiety, such as a fatty acid, is described. The complex has an enhanced absorption in the gastrointestinal tract, particularly the lower gastrointestinal tract. The complex, and compositions and dosage forms prepared using the complex, provide for absorption by the body of iron through a period of ten to twenty-four hours, thus enabling a true once-daily dosage form for iron.
Owner:ALZA CORP
Delivery and controlled release of encapsulated lipophilic nutrients
ActiveUS20090061048A1Reduces aftertasteImprove bioavailabilityBiocideHydroxy compound active ingredientsPh controlFish oil
A complex coacervate delivery system is provided which encapsulates lipophilic nutrients such as, for example, fish oils high in omega-3 fatty acids. The complex coacervate delivery system protects the lipophilic nutrient from degradation, e.g., oxidation and hydrolysis, and also reduces or eliminates the unpleasant taste and odor of the lipophilic nutrient. The complex coacervate delivery system upon ingestion is operative to substantially release the lipophilic nutrient in the lower gastrointestinal tract in a pH-controlled manner. The complex coacervate delivery system may be included in a food or beverage product having a pH value within the range of about 1.5 to about 5.0.
Owner:PEPSICO INC
Release of statins in the intestine
InactiveUS20100055173A1Reduced food effect on the releaseBiocideCapsule deliveryIntestinal structureLower Gastrointestinal Tract
The present invention provides a controlled absorption formulation in which modified release of the active ingredient preferentially occurs in the lower gastrointestinal tract, including the colon. The formulation supports a significantly higher bioavailability of the active ingredient in the body of the subject than that can be achieved from the currently used conventional formulation, such that therapeutically significant plasma levels of statin are maintained for an extended period after administration. The formulation preferably features a core, a subcoat surrounding the core comprising at least one water soluble hydrophilic carrier and an outer coating. The core is optionally and preferably in the form of a tablet.
Owner:DEXCEL PHARMA TECH
Gastric retentive oral dosage form with restricted drug release in the lower gastrointestinal tract
InactiveUS20110301129A1Minimizes variabilityIncreasing and decreasing drug loadingAntibacterial agentsBiocideDrug release rateErosion rate
Owner:DEPOMED SYST INC
Combination growth factor therapy and cell therapy for treatment of acute and chronic diseases of the organs
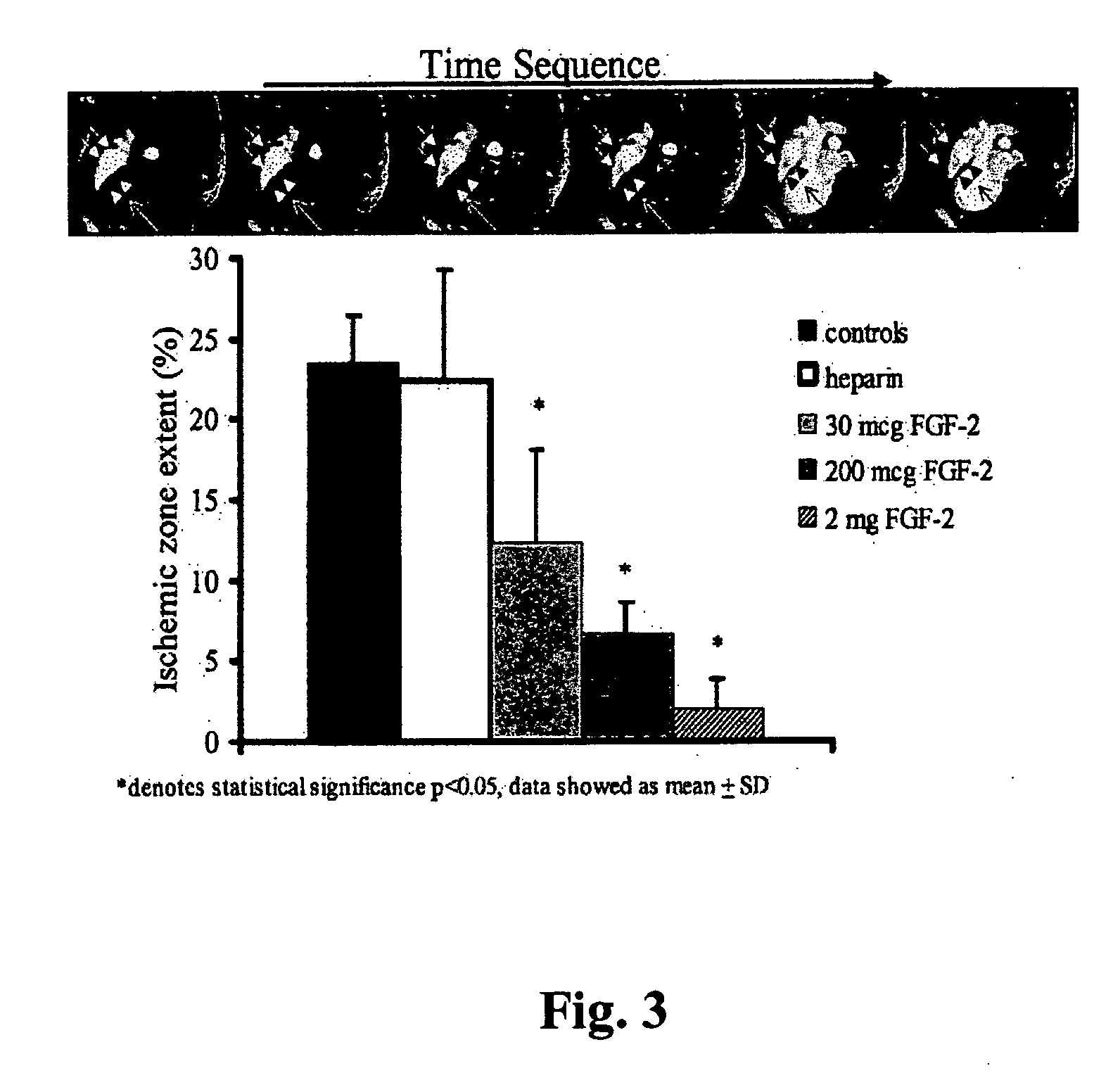
Acute and chronic diseases of the organs are treated using a rational, multi-tier approach. A patient is pretreated with growth factor proteins or gene therapy, followed by the administration of adult stem cells or other cell therapy. The patient can be a fetus treated in utero or removed from the womb. The progress of treatment is monitored by ultrasound, MRI, CAT scan, cardiac echo, EEG, EKG, EMG or blood tests, with growth factor treatment and / or stem cell administration adjusted according to the results of the monitoring or clinical status of the patient. Organ disease is also treated by a method that comprises administration of a therapeutically effective amount of a growth factor protein by oral inhalation or intranasal therapy. Diseases affecting organs such as the brain, spinal cord, pancreas, liver, kidney, muscle, heart and upper and lower gastrointestinal tracts are treated using the present method. A device for treatment is disclosed. CPK is utilized to assess the treatment of muscle disease.
Owner:FRANCO WAYNE P
Localized controlled absorption of statins in the gastrointestinal tract for achieving high blood levels of statins
InactiveUS20060251720A1Reduce the impactIncrease volumeBiocideMetabolism disorderParticulatesWater insoluble
The present invention relates to a localized controlled absorption formulation of a statin in which rapid release of the active ingredient preferentially occurs in the lower gastrointestinal tract including the colon. The formulation provides significantly higher blood level concentration and bioavailability of the active ingredient in the body of a subject as compared to the bioavailability achieved from the currently available conventional formulations The blood levels are maintained for a significantly longer period of time as compared with currently available conventional formulations. The formulation preferably includes a core, over which an outer coating is layered. The core preferably includes a burst controlling agent and optionally a disintegrant. The outer coating includes a water insoluble polymer and at least one water permeable agent allowing entry of water into said core, the water permeable agent comprising hydrophilic particulate matter. The core is preferably in the form of a tablet.
Owner:DEXCEL PHARMA TECH
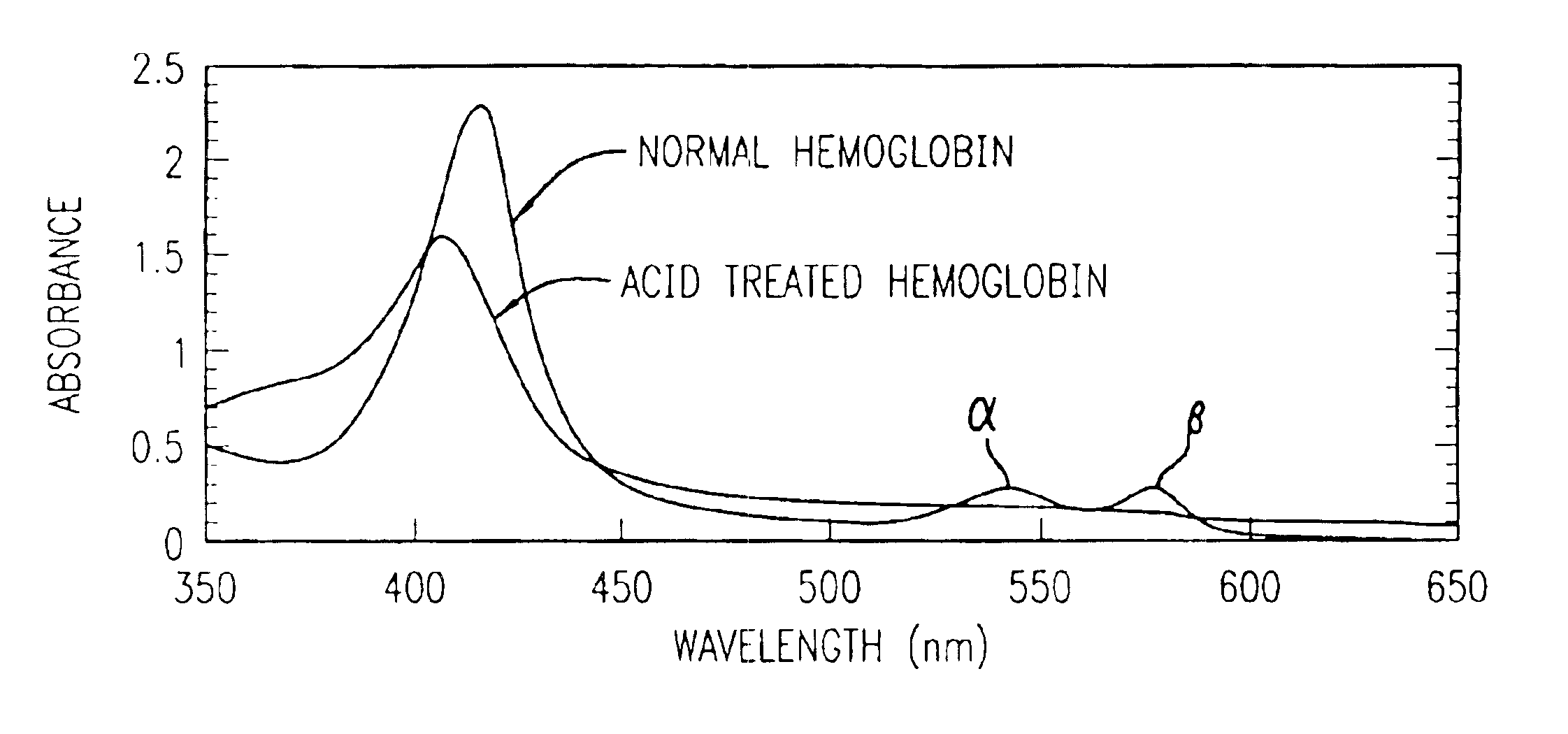
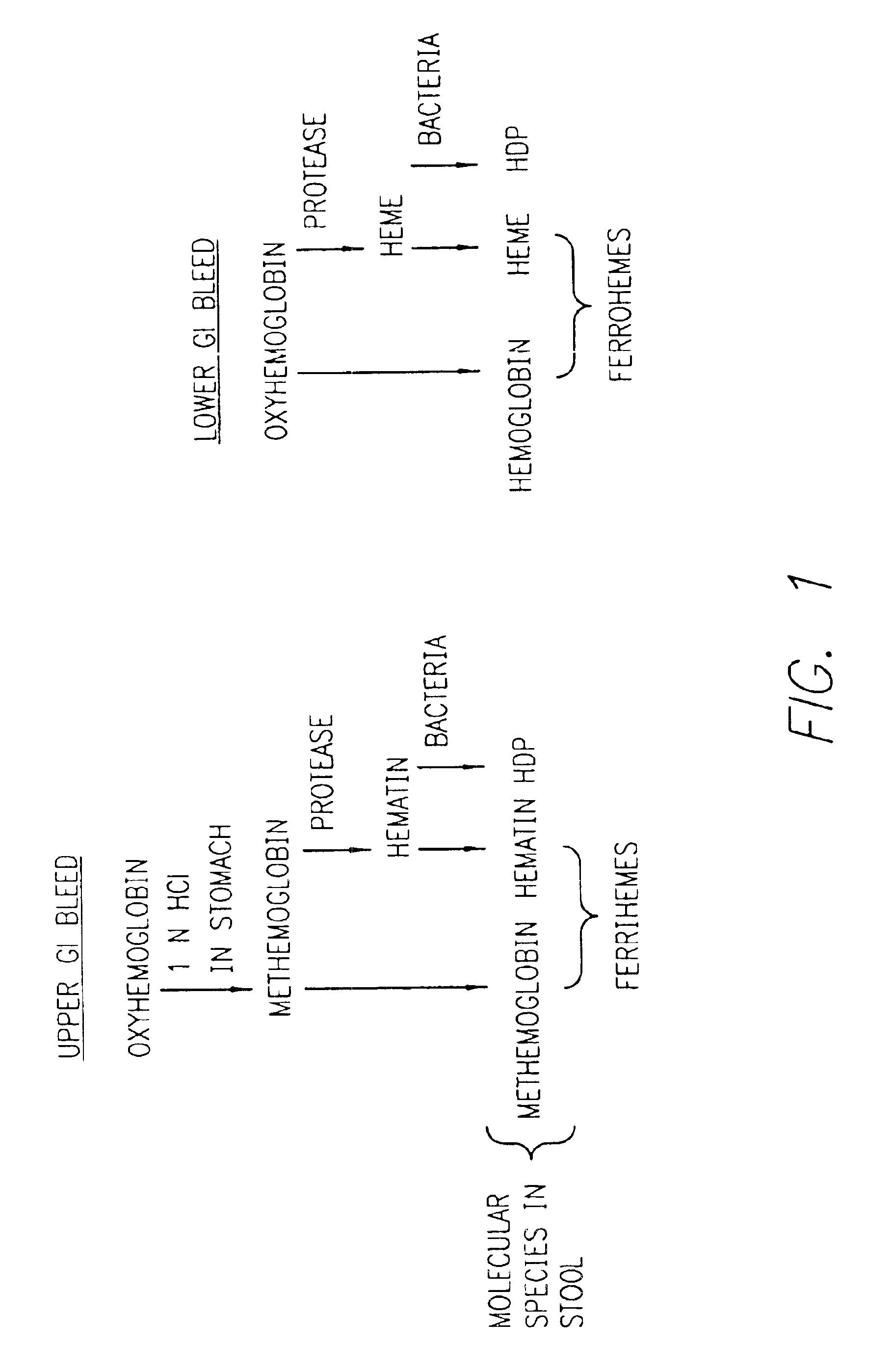
Method for determining location of gastrointestinal bleeding
InactiveUS6844195B2Rapid and economicalPerformance requirementAnalysis using chemical indicatorsPreparing sample for investigationFecesLower Gastrointestinal Tract
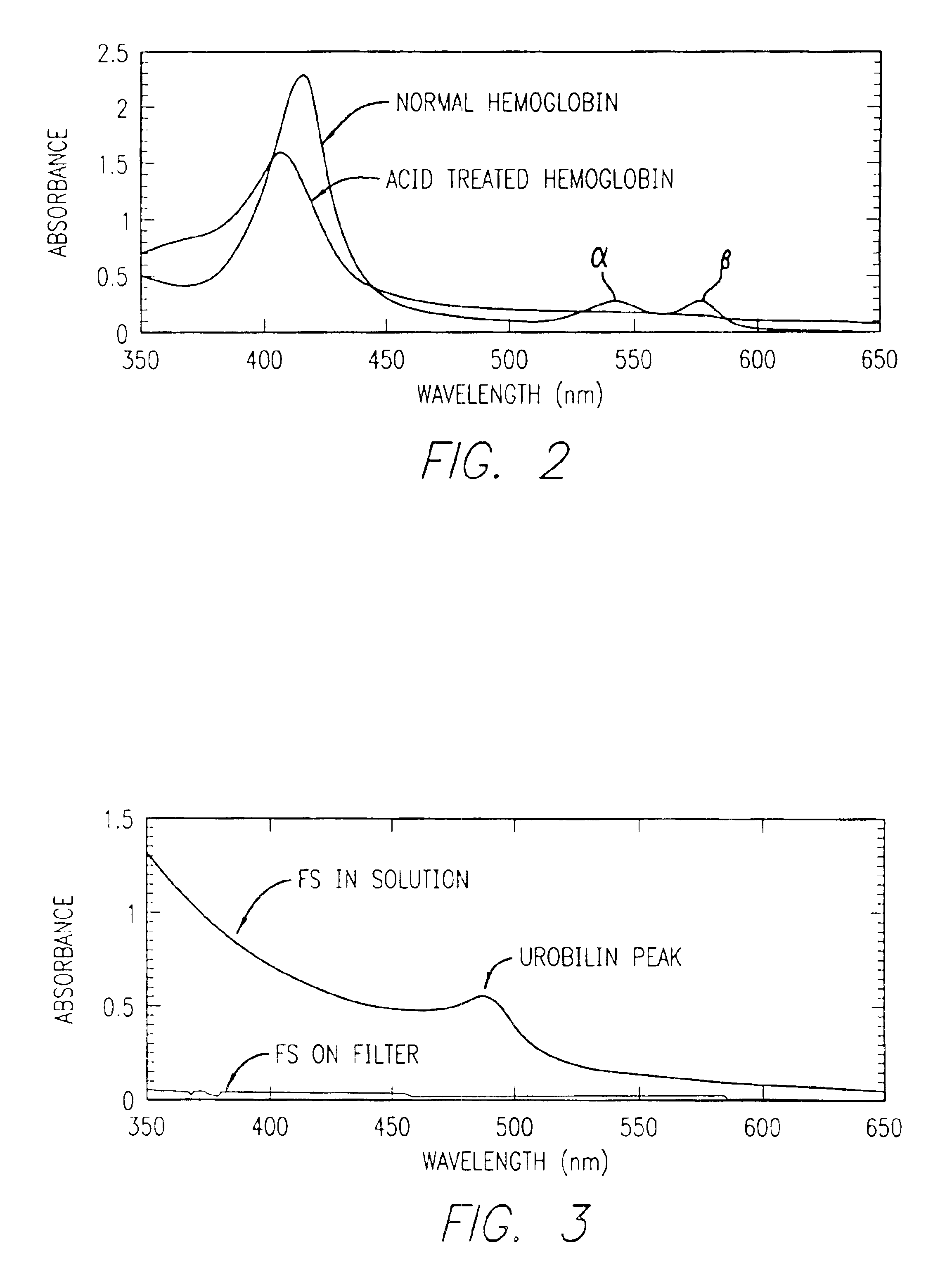
A method for determining if blood in a stool sample originated from the upper or lower gastrointestinal tract. This includes a method for purifying and concentrating hemoglobin and its products from a stool sample to allow a simple and sensitive spectrophotometric analysis. A rapid, noninvasive determination of whether the blood originated from an upper gastrointestinal or lower gastrointestinal site is made on the basis of changes in the absorption spectra of hemoglobin that occur when hemoglobin is exposed to a highly acidic environment.
Owner:WESTERN RES
Methods and compositions for detecting gastrointestinal and other cancers
ActiveUS8415100B2Significant positive effectSugar derivativesMicrobiological testing/measurementLower Gastrointestinal TractNucleotide
Owner:CASE WESTERN RESERVE UNIV
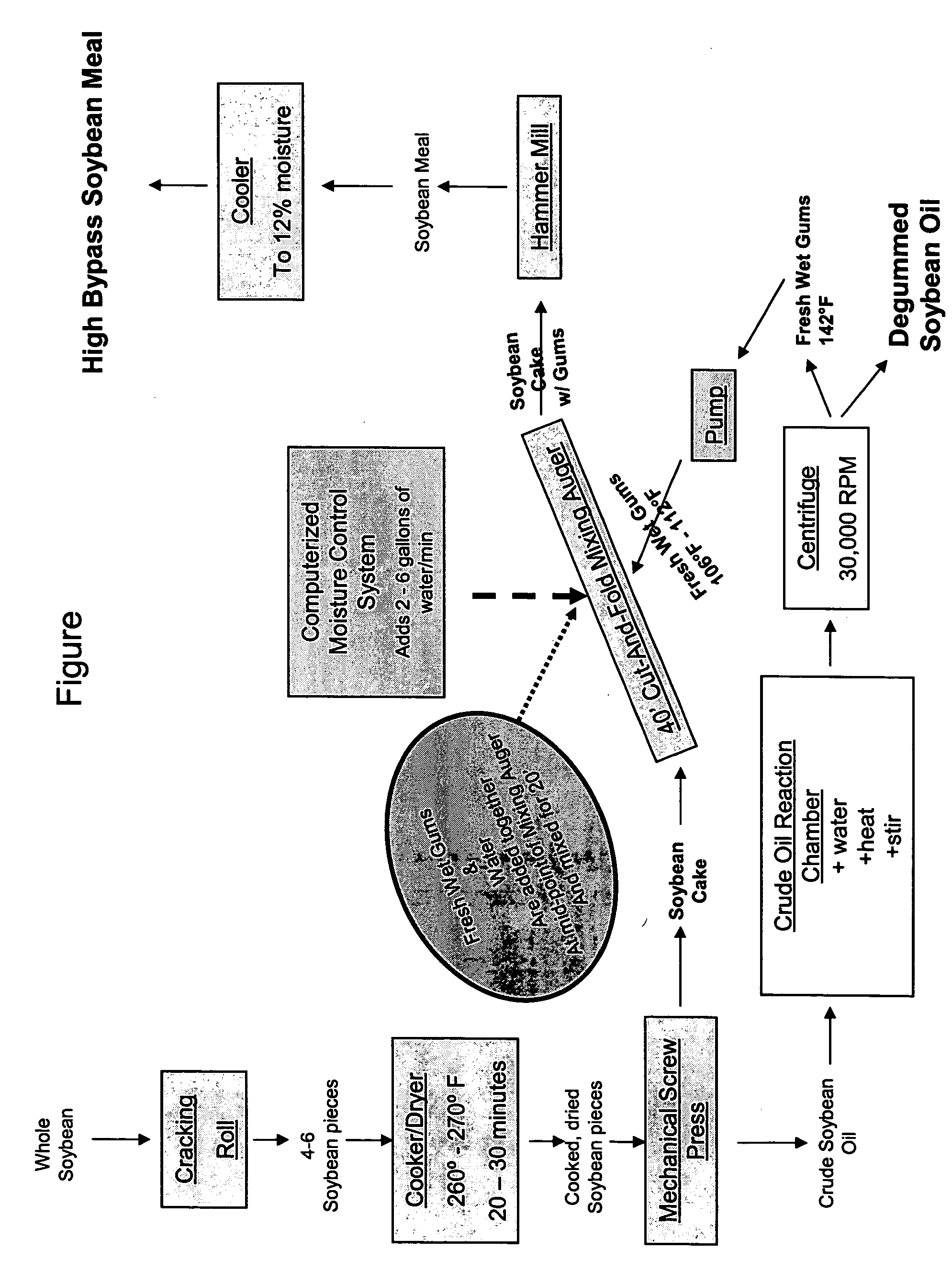
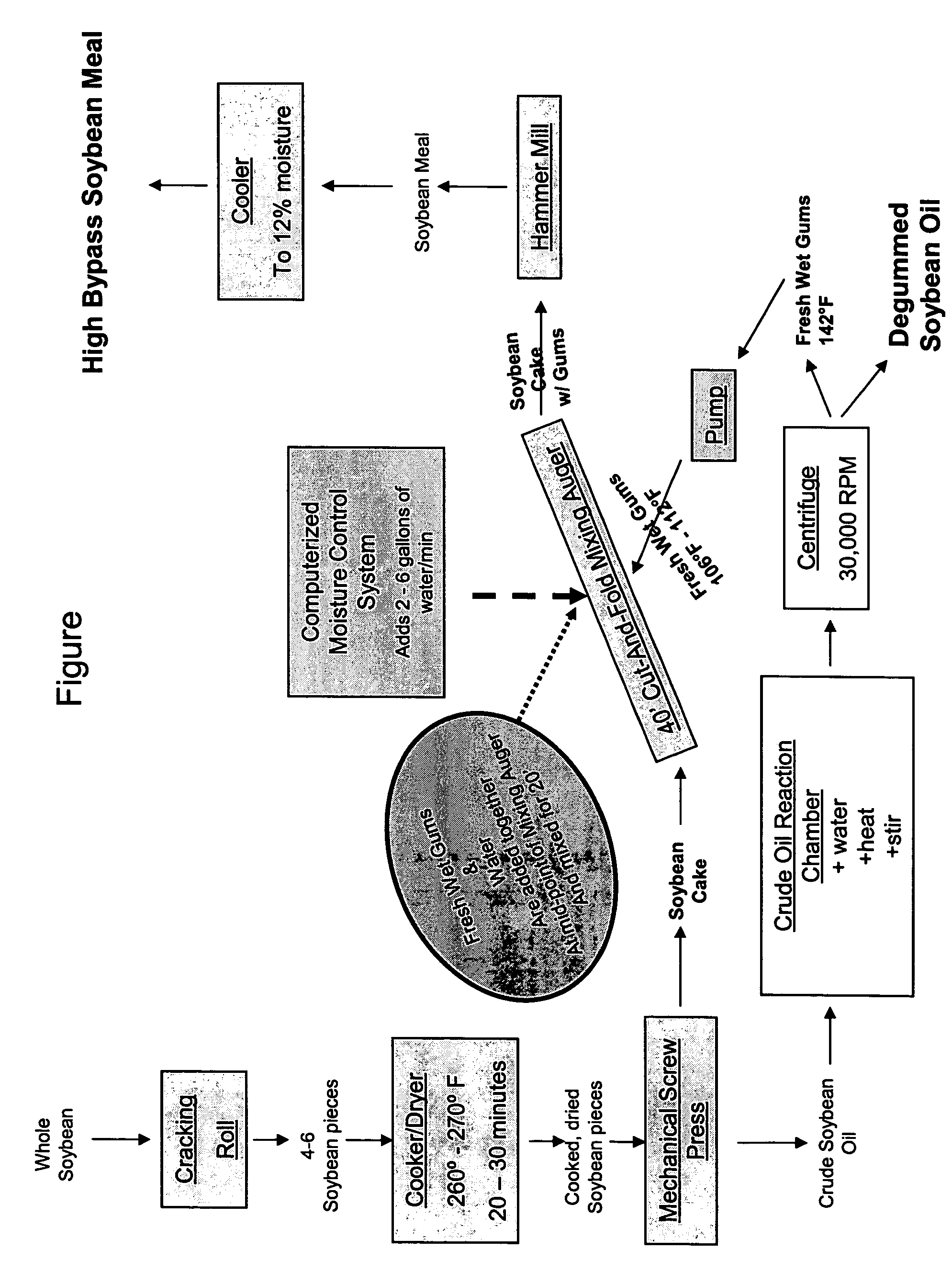
Method for manufacturing animal feed, method for increasing the rumen bypass capability of an animal feedstuff and animal feed
ActiveUS20050255145A1Enhance bypass natureAvoid damageFood processingConfectioneryRuminant animalLower Gastrointestinal Tract
An animal feed that comprises a feedstuff and a coating, where the coating increases the amount of the feedstuff that passes through the rumen without being degraded by the rumen microflora, thereby delivering a larger portion of that feedstuff's associated preformed protein, and the essential amino acids comprising that protein, to the lower gastrointestinal tract. A process for making an animal feed, where the animal feed has enhanced rumen bypass nature of feed ingredients and their associated nutrients, particularly preformed protein and the amino acids that comprise the protein. Methods of increasing the rumen bypass of phosphatidylcholine, methods of increasing the vitamin E value of a feedstuff, methods for increasing rumen escape of the protein and amino acids in a ruminant animal.
Owner:GRAIN STATES SOYA
Endoscopic overtube
InactiveUS20100331625A1Prevents insufflation leakagePrevent leakageSurgeryEndoscopesLower Gastrointestinal TractSurgical department
An over-tube was developed for receiving and guiding endoscopic instruments into a patient's anus, through the colon, and into the peritoneum. The over-tube has a flexible sheath adapted for insertion into the anus through the colon and out the peritoneum of a patient. A stiffened region is used to protect the colonic wall from injury during insertion of medical instruments through the patient's lower gastrointestinal tract. The proximal end of the over-tube is surrounded by two narrow inflatable balloons on either side of the colotomy to keep the device in place with its lumen open into the peritoneum and further prevent leakage of insufflation. The device may comprise two channels, one for duel channel endoscopes and larger medical instruments or single lumen endoscopes, and an additional channel for surgical instruments. A distal valve prevents insufflation leakage, making the device especially useful in NOTES surgeries.
Owner:UNIV OF SOUTH FLORIDA
Device and Method for Detecting the Presence of Hemoglobin in a Biological Sample
InactiveUS20080113382A1Useful in detectionResistant to breakdownBioreactor/fermenter combinationsBiological substance pretreatmentsFecesLower Gastrointestinal Tract
A device and method for detecting the presence of hemoglobin in a biological sample, more particularly, the presence of blood in a fecal sample as an indicator of upper or lower gastrointestinal tract bleeding.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Enteric-coated sodium metabisulfite livestock feed additive for vomitoxin detoxification
A livestock feed supplement in which a core particle containing sodium metabisulfite and at least one binder is enrobed with an enteric coating, wherein the thickness and composition of the coating protects the sodium metabisulfite from decomposition to sulfur dioxide in an aqueous acid stomach environment. Also disclosed are a method of delivering sodium metabisulfite to the lower gastrointestinal tract of an animal, and a method of delivering an antidote to relieve the toxic effect of vomitoxin in an animal, by administering to the animal the livestock feed supplement.
Owner:PROVIMI NORTH AMERICA
Oral formulations
InactiveUS20120093939A1Powder deliverySalicyclic acid active ingredientsLower Gastrointestinal TractImmediate release
Disclosed are pharmaceutical compositions comprising immediate release and sustained release formulations of 5-aminosalicylic acid, or a pharmaceutically acceptable salt or ester thereof, and / or N-acetylcysteine, or a pharmaceutically acceptable salt or ester thereof, for release in the lower gastrointestinal tract.
Owner:ALTHEUS THERAPEUTICS
Delivery and Controlled Release of Encapsulated Lipophilic Nutrients
InactiveUS20160206561A1Improve bioavailabilityImprove efficacyOrganic active ingredientsDispersion deliveryPh controlFish oil
A complex coacervate delivery system is provided which encapsulates lipophilic nutrients such as, for example, fish oils high in omega-3 fatty acids. The complex coacervate delivery system protects the lipophilic nutrient from degradation, e.g., oxidation and hydrolysis, and also reduces or eliminates the unpleasant taste and odor of the lipophilic nutrient. The complex coacervate delivery system upon ingestion is operative to substantially release the lipophilic nutrient in the lower gastrointestinal tract in a pH-controlled manner. The complex coacervate delivery system may be included in a food or beverage product having a pH value within the range of about 1.5 to about 5.0.
Owner:PEPSICO INC
Protection of microbial cells from acidic degradation
InactiveUS20140023693A1Increase weightImprove food utilizationBiocideSkeletal disorderCelluloseHigh survival rate
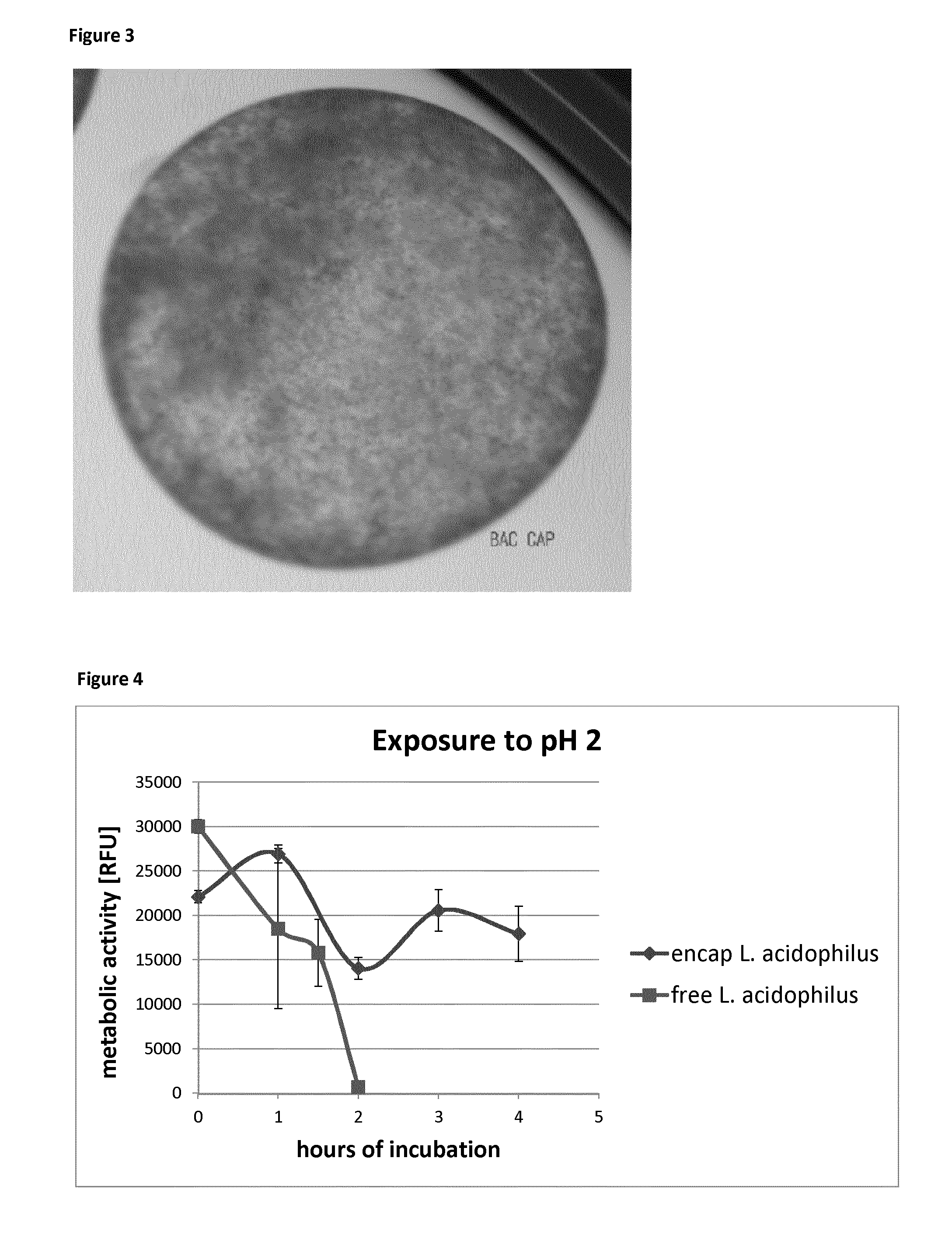
A simple cellulose sulphate based microencapsulation technology has been applied to encapsulate bacterial or other microbial cells, which produce and release digestive enzymes and thereby provides an acid resistant shelter for these microbial cells. Surprisingly, the resulting spheres were found to provide sufficient protection for encapsulated cells from treatment with aqueous acidic solutions. Thereby the cellulose sulphate microencapsulated cells, such as probiotics are now enabled to survive passage, for example, through the stomach after consumption by a human or animal with a higher survival rate than those not within a microcapsule. After passing the stomach these cells are delivering products produced by them, e.g. enzymes or other nutrition factors. This technology therefore proves to be very useful in providing digestive or otherwise beneficial enzymes and / or of living microbial cells, into the lower gastrointestinal tract, where they could confer their health benefit to the host.
Owner:AUSTRIANOVA SINGAPORE PTE
Controlled absorption of statins in the intestine
InactiveUS20090196889A1Reduce the impactSure easyBiocideAnimal repellantsIntestinal structureLower Gastrointestinal Tract
The present invention provides a controlled absorption formulation in which modified release of active ingredient preferentially occurs in the lower gastrointestinal tract, including the colon. The formulation supports a significantly higher bioavailability of the active ingredient into the body of the subject than can be achieved from the currently used conventional formulation, such that therapeutically significant plasma levels of statin are maintained for an extended period after administration. The formulation preferably features a core over which an outer coating is layered. The core is optionally and preferentially in the form of a tablet.
Owner:DEXCEL PHARMA TECH
Compositions and dosage forms for enhanced absorption of gabapentin and pregabalin
A complex comprised of metformin and a transport moiety, such as a fatty acid, is described. The complex has an enhanced absorption in the gastrointestinal tract, particularly the lower gastrointestinal tract. The complex, and compositions and dosage forms prepared using the complex, provide for absorption by the body of the drug through a period of ten to twenty-four hours, thus enabling a once-daily dosage form for metformin.
Owner:ALZA CORP
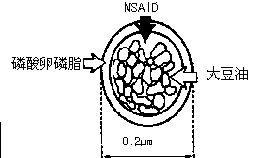
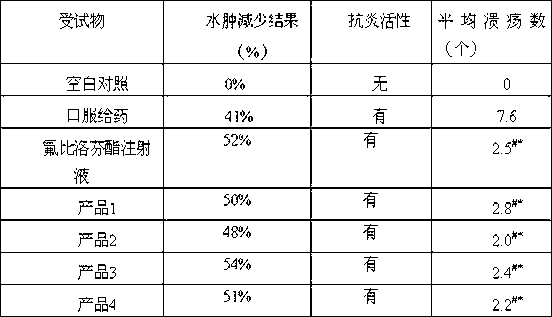
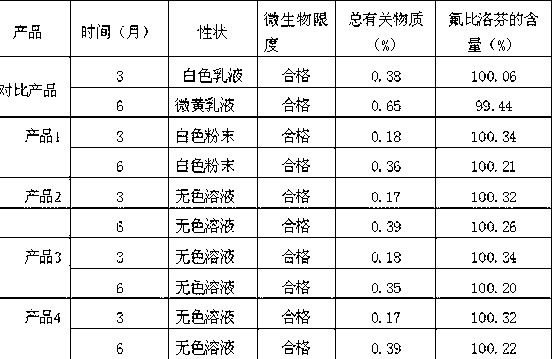
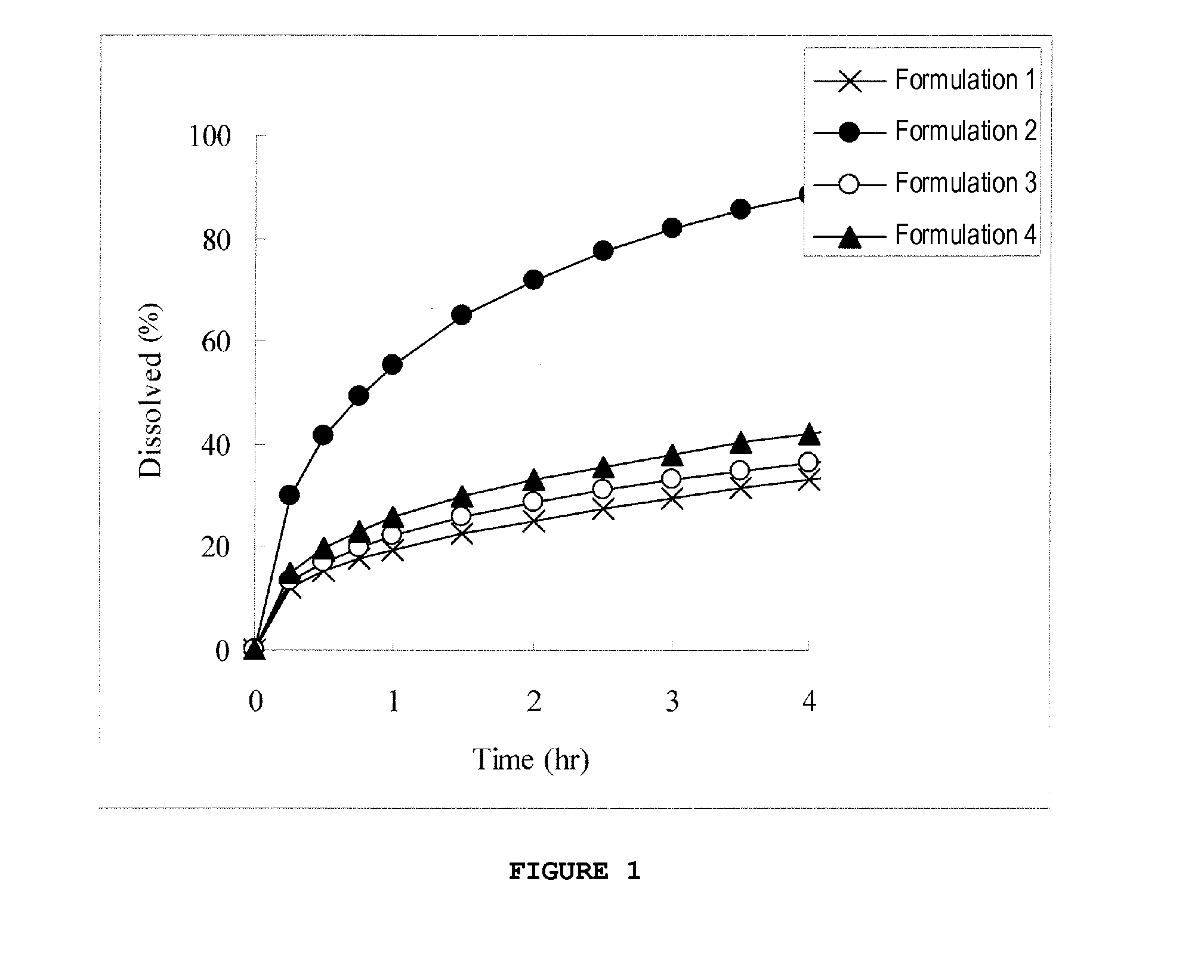
Novel 2-(2-fluorine-4biphenyl)-propionic acid pharmaceutical composition
ActiveCN103301101AReduce gastrointestinal adverse reactionsIndustrial applicabilityOrganic active ingredientsNervous disorderArgininePropionine
The invention provides a novel 2-(2-fluorine-4biphenyl)-propionic acid pharmaceutical composition. Flurbiprofen and basic amino acid arginine or lysine form a pharmaceutical composition solution. The composition can be administrated in an injection or oral administration manner and can also be further administrated in a freeze-drying manner. Compared with a flurbiprofen axetil injection, a flurbiprofen composition not only does not influence the antipyretic, anti-inflammation and analgestic effects of the flurbiprofen, but also has the advantages of simple technology, low cost, high quality, stable long shelf life and convenience in quality control, storage and transportation; moreover, compared with a conventional oral preparation, the flurbiprofen composition has the same low gastrointestinal tract adverse reaction as the flurbiprofen axetil injection.
Owner:南京星福星医药科技有限公司
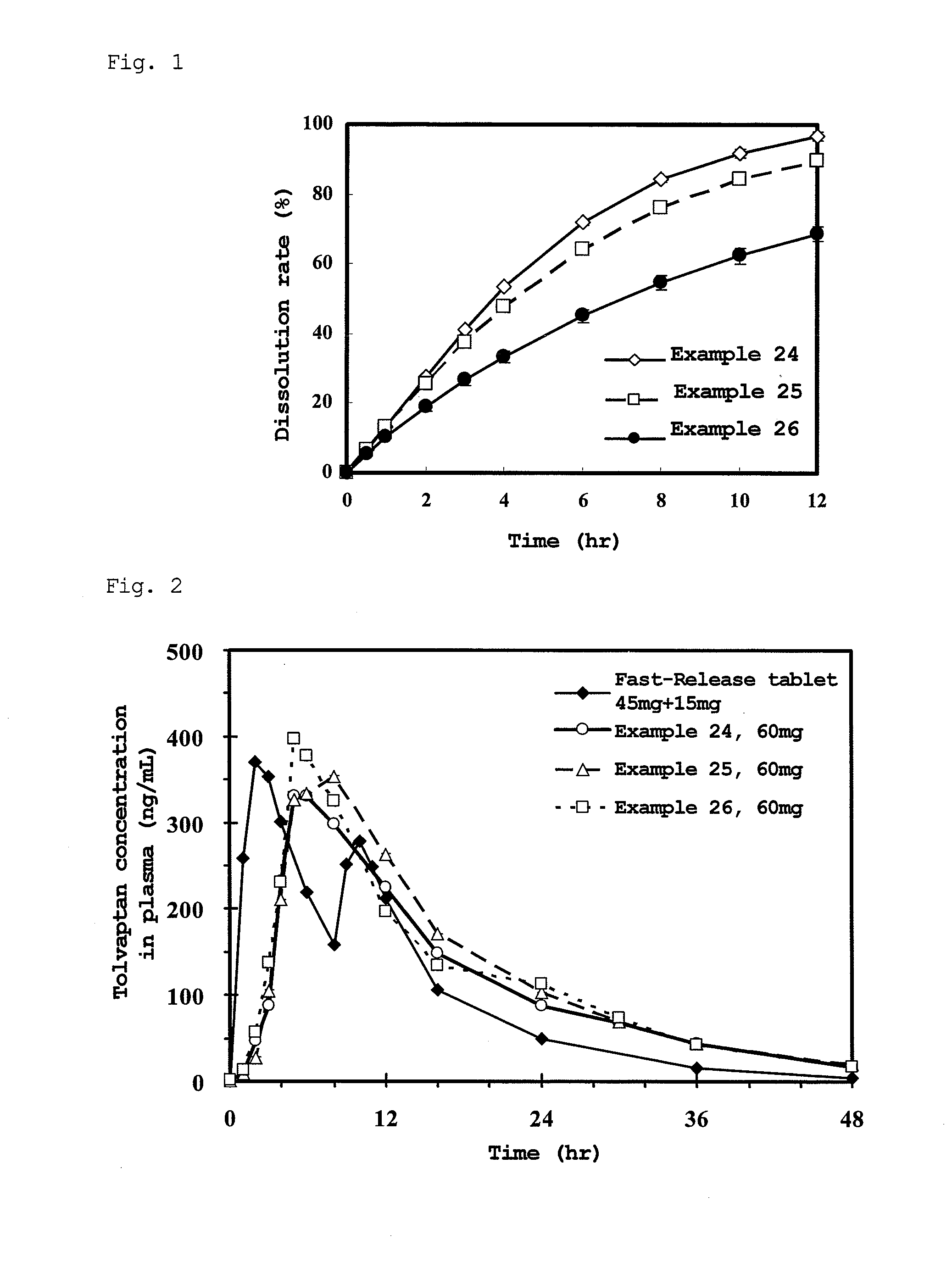
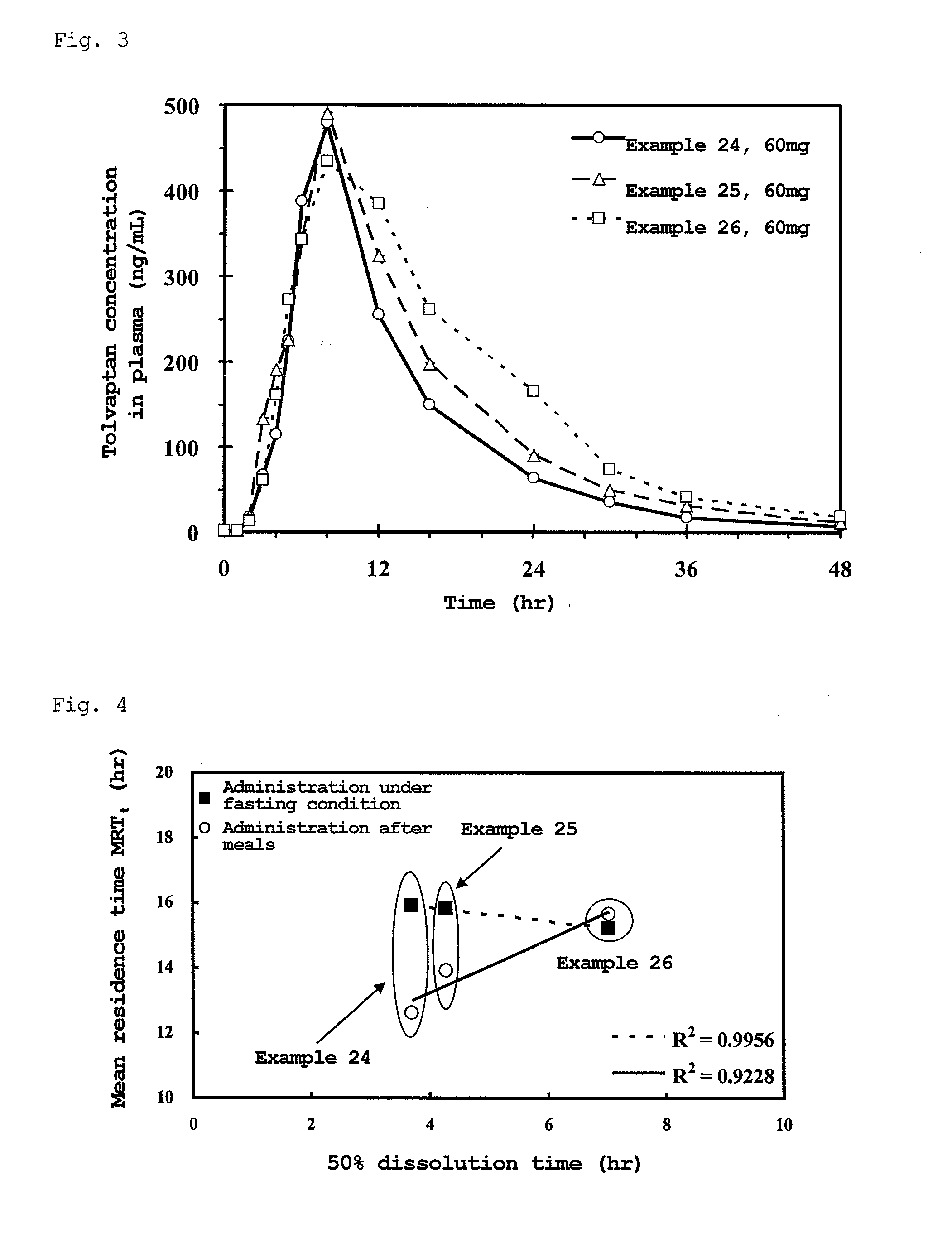
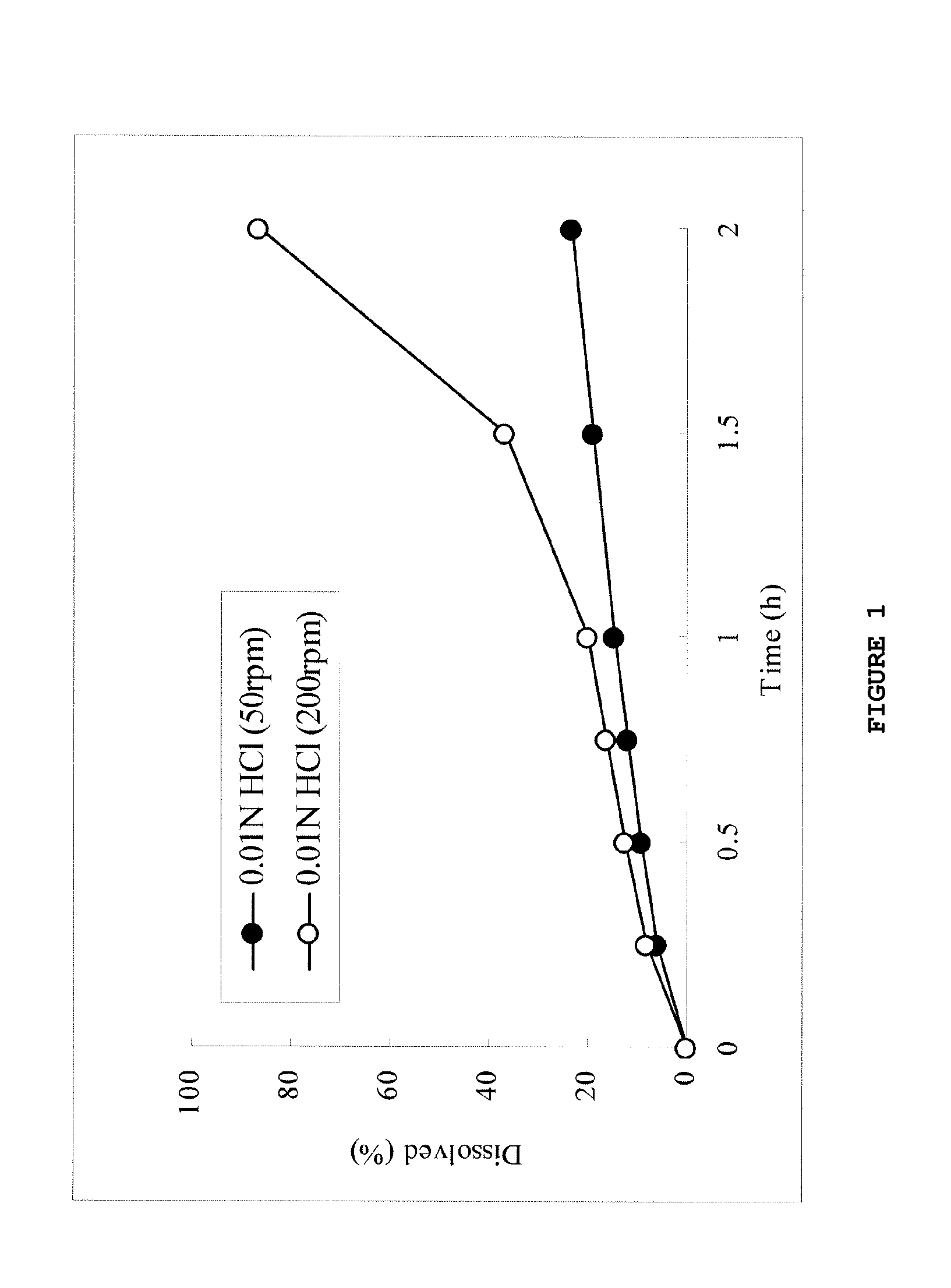
Sustained-release solid preparation for oral use
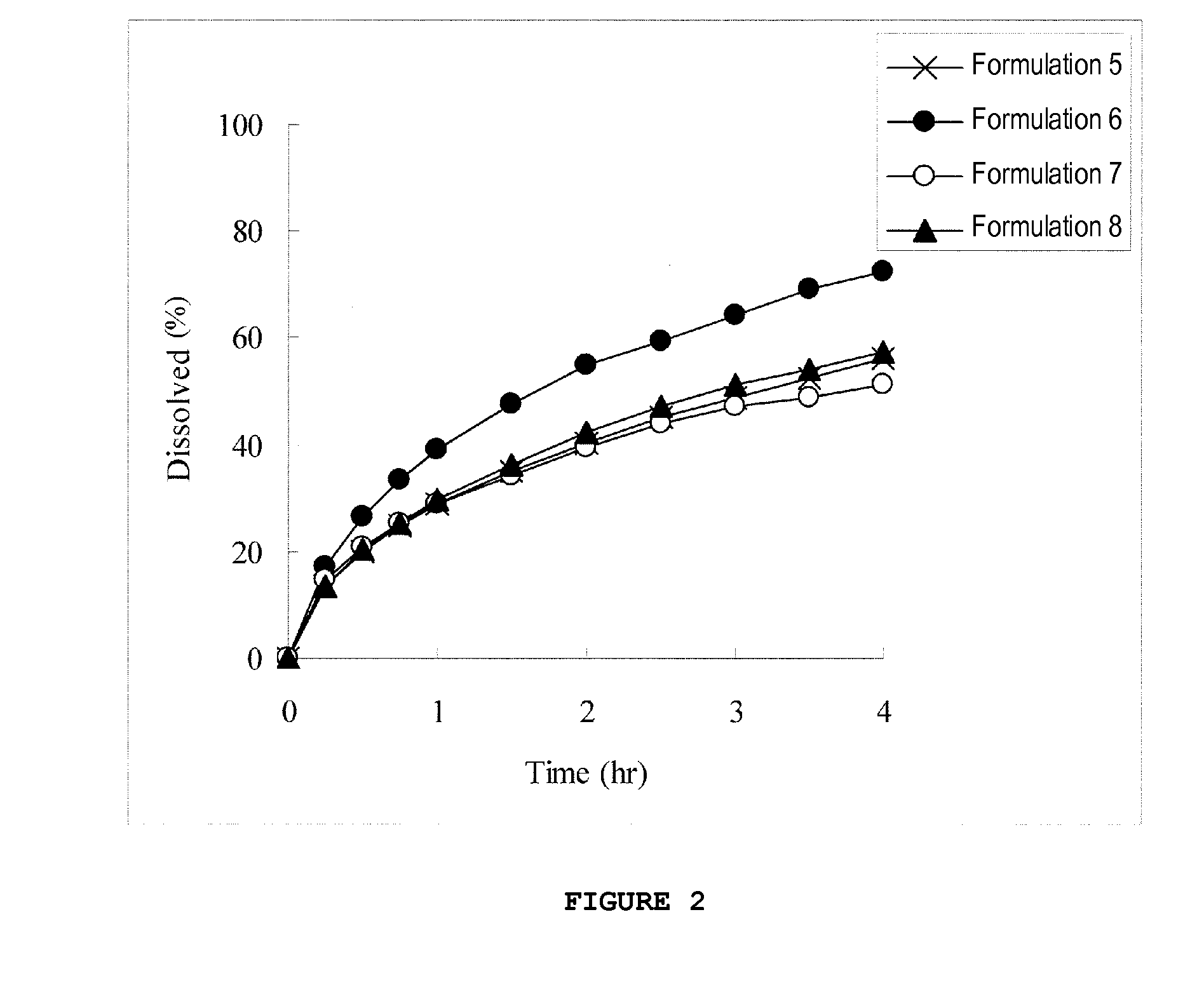
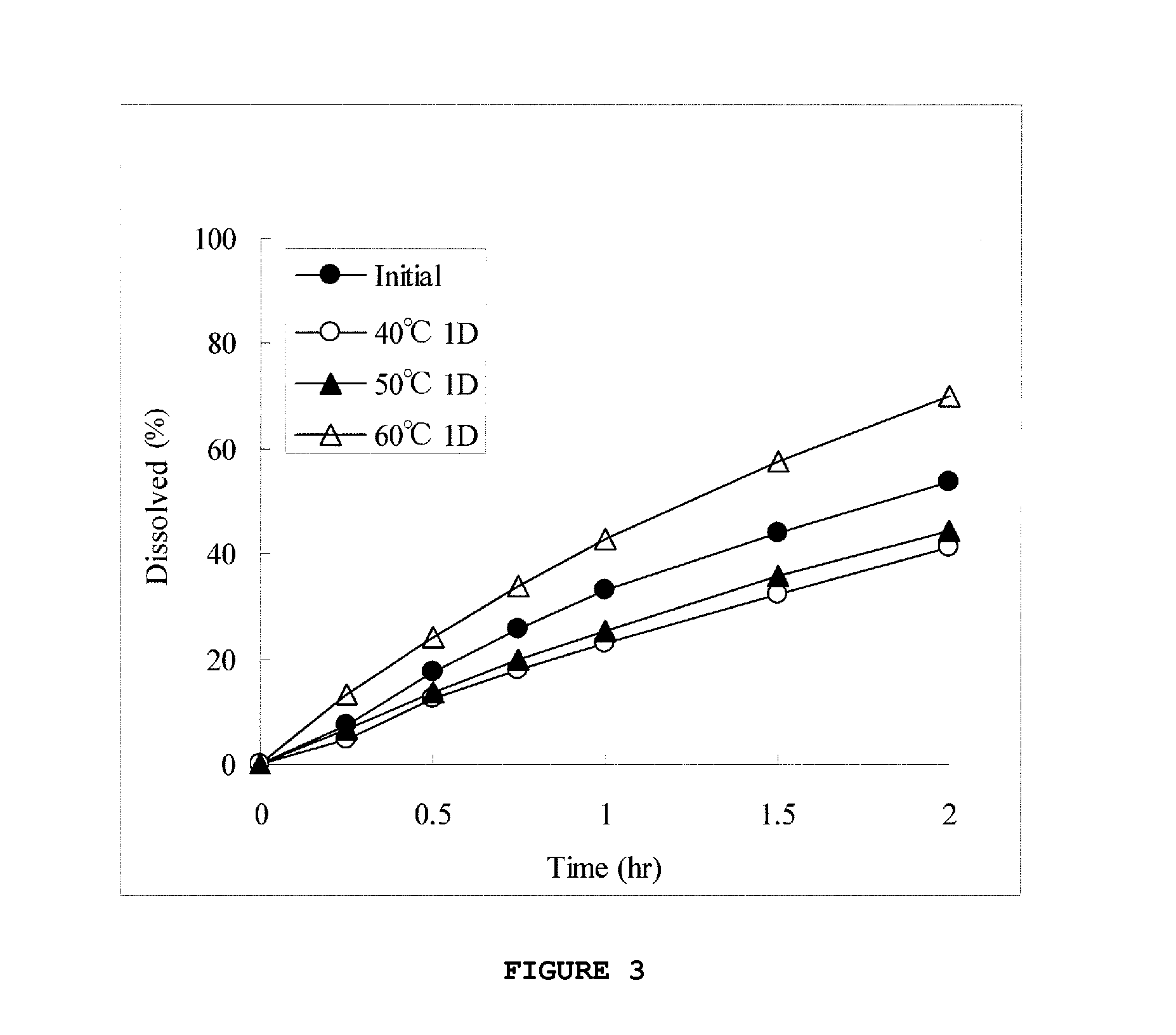
InactiveUS20130012535A1High strengthAvoid dose dumpingPowder deliveryBiocideSustained release pelletsLower Gastrointestinal Tract
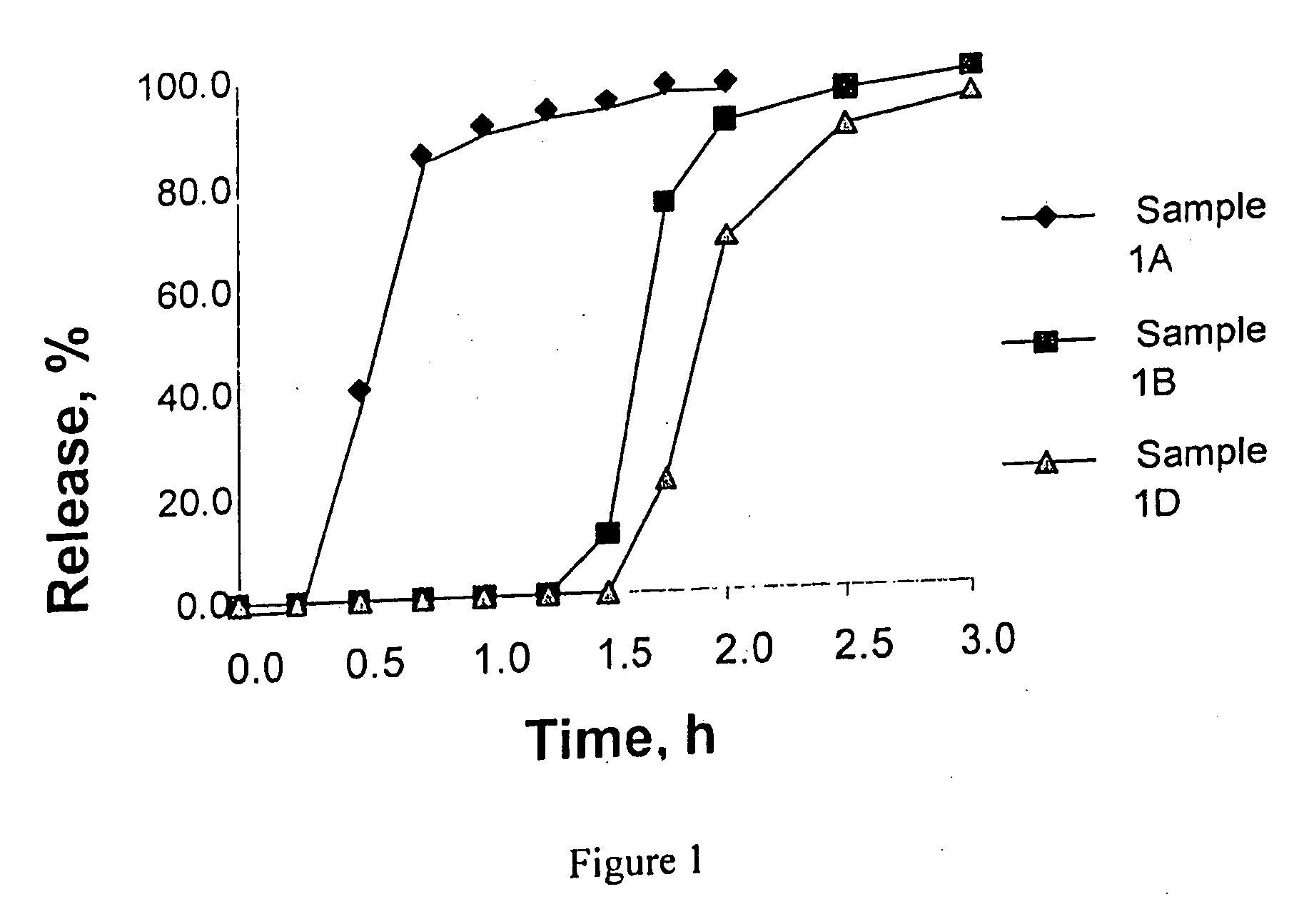
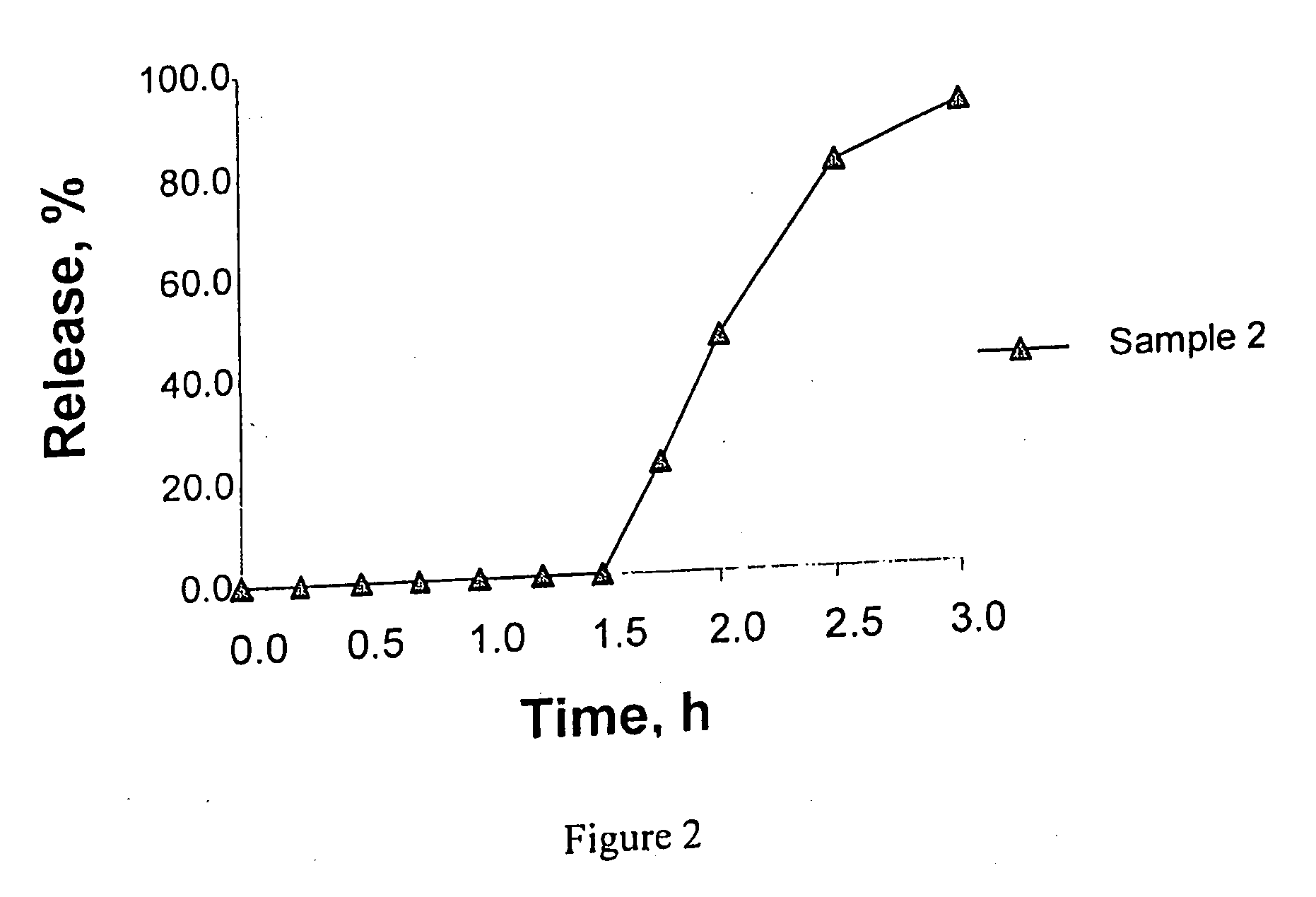
It is intended to avoid dose dumping of a drug and improve the dissolution properties of the drug in the lower gastrointestinal tract, and thereby provide a sustained-release pellet preparation for oral administration that reliably exhibits its main pharmacological effect when orally administered once or twice a day. The present invention provides a sustained-release preparation obtained by mixing of (A) a pharmacologically active drug, (B) hydroxypropyl methylcellulose acetate succinate, (C) a plasticizer, and (D) polyethylene glycol followed by extrusion granulation.
Owner:DAIICHI SANKYO CO LTD
System for release in lower gastrointestinal tract
InactiveUS20050249800A1Control ratePowder deliveryCapsule deliveryLower Gastrointestinal TractCompound (substance)
A system whereby a substance which is orally taken and to be delivered into the lower digestive tract is selectively delivered into the lower digestive tract. More particularly, a system which makes it possible to surely and quickly deliver the aimed substance to the lower digestive tract without being affected by pH change in the digestive tract due to change in bacterial flora. Compositions disintegrating in the lower digestive tract characterized by containing a compound <A>, which has a molecular weight of 1000 or less and has a disulfide bond, and a polymer <B>, which has a molecular weight exceeding 1000 and is digested by enteric bacteria and / or undergoes softening, swelling or dissolution due to a decrease in pH; molded products with the use of these compositions; and preparations with the use of these molded products.
Owner:KUDO YUMIO +2
Compositions and dosage forms for enhanced absorption of gabapentin and pregabalin
A complex comprised of gabapentin or pregabalin and a transport moiety, such as an alkyl sulfate, is described. The complex has an enhanced absorption in the gastrointestinal tract, particularly the lower gastrointestinal tract. The complex, and compositions and dosage forms prepared using the complex, provide for absorption by the body of the drug through a period of ten to twenty-four hours, thus enabling a once-daily dosage form for gabapentin or pregabalin.
Owner:ALZA CORP
Matrix-type pharmaceutical solid preparation
InactiveUS20100233265A1Easy to handleEasy to takeBiocidePowder deliveryLower Gastrointestinal TractUpper gastrointestinal
The present invention aims to provide a matrix-type solid preparation that has high-level release controllability for suppressing drug release in the upper gastrointestinal tract and accelerating drug release in the lower gastrointestinal tract, and that solves of all the above drawbacks caused by combining a plasticizer. The present invention provides a matrix-type pharmaceutical solid preparation that contains: (a) a methacrylic acid-based enteric polymer; and (b) a sugar and / or a sugar alcohol, wherein 1 g of the sugar and / or the sugar alcohol can be dissolved in not more than 4 g of water at a water temperature of 20 to 25° C.
Owner:OTSUKA PHARM CO LTD
Method for manufacturing animal feed, method for increasing the rumen bypass capability of an animal feedstuff and animal feed
ActiveUS7297356B2Improve abilitiesRaise the ratioFood processingConfectioneryRuminant animalLower Gastrointestinal Tract
An animal feed that comprises a feedstuff and a coating, where the coating increases the amount of the feedstuff that passes through the rumen without being degraded by the rumen microflora, thereby delivering a larger portion of that feedstuff's associated preformed protein, and the essential amino acids comprising that protein, to the lower gastrointestinal tract. A process for making an animal feed, where the animal feed has enhanced rumen bypass nature of feed ingredients and their associated nutrients, particularly preformed protein and the amino acids that comprise the protein. Methods of increasing the rumen bypass of phosphatidylcholine, methods of increasing the vitamin E value of a feedstuff, methods for increasing rumen escape of the protein and amino acids in a ruminant animal.
Owner:GRAIN STATES SOYA
Sustained-release solid preparation for oral use
InactiveUS20130005763A1Increase ratingsAvoid dose dumpingBiocidePill deliveryCarmellose SodiumLower Gastrointestinal Tract
It is intended to avoid dose dumping of a drug and improve the dissolution properties of a drug in the lower gastrointestinal tract, and thereby provide a sustained-release solid preparation for oral administration that reliably exhibits its main pharmacological effect when orally administered once or twice a day. The present invention provides a sustained-release solid preparation containing (A) a pharmacologically active drug, (B) carboxyvinyl polymer, (C) povidone, and (D) carmellose sodium, xanthan gum, or sodium carboxymethyl starch.
Owner:DAIICHI SANKYO CO LTD
Sustained-release solid preparation for oral use
InactiveUS20130004550A1Favorable tablet strengthAvoid dose dumpingPowder deliveryBiocideAcetic acidLower Gastrointestinal Tract
It is intended to avoid dose dumping of a drug and improve the dissolution properties of the drug in the lower gastrointestinal tract, and thereby provide a sustained-release matrix preparation for oral administration that reliably exhibits its main pharmacological effect when orally administered once or twice a day. The present invention provides a sustained-release preparation obtained by mixing of (A) a pharmacologically active drug, (B) hydroxypropyl methylcellulose acetate succinate having a median size (D50) of 40 μm or smaller, (C) a cellulose derivative, and (D) a saccharide or a nonionic water-soluble polymer followed by molding.
Owner:DAIICHI SANKYO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com