System for release in lower gastrointestinal tract
a technology for releasing systems and gastrointestinal tracts, applied in the direction of powder delivery, medical preparations, capsule delivery, etc., can solve the problems of unfailingly realizing colon-specific delivery of drugs, and difficult to unfailingly deliver drugs colon-specifically
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
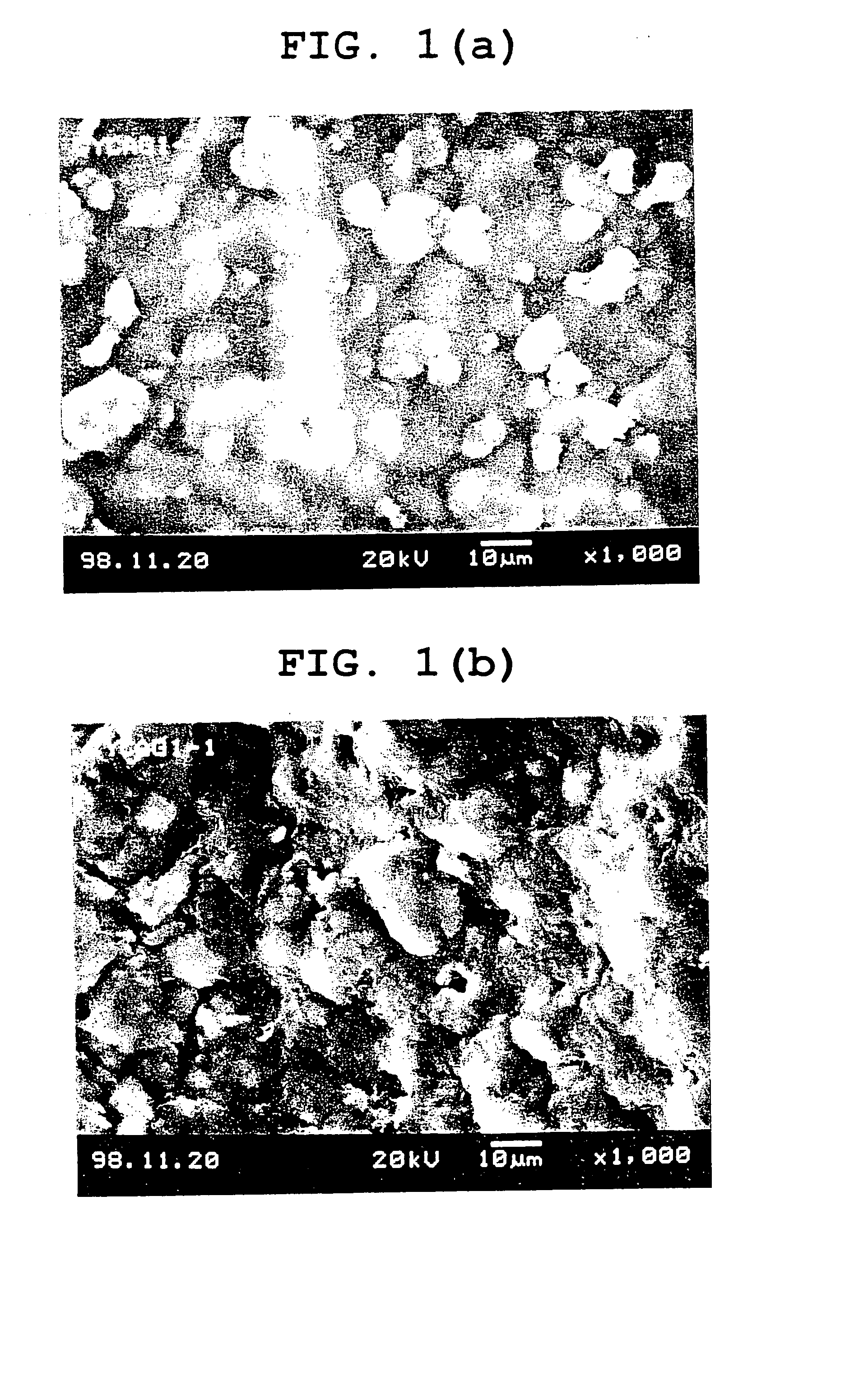
[0115] 50 ml of water was added to 1 g of agar, 1 g of cystine, and 1 g of glycerol, and the mixture was stirred at 90° C. to dissolve the agar. After the temperature of the resultant suspension was decreased to 70° C., 1.5 g of gelatin was added and the mixture was stirred to dissolve. Further, a solution of 1.5 g of chitosan (Chitosan LL (registered trademark), viscosity (0.5%, 20° C.); 20 cps or more, Yaizu Suisan), and 0.5 g of acetic acid in 25 ml of water was added thereto and stirred to obtain a uniform suspension.
[0116] 7 ml of this suspension is spread in a dish of 9.3 mm in inner diameter and dried to prepare a cast film (about 60 μm in film thickness).
example 2
[0117] 50 ml of water was added to 1 g of agar, 1 g of cystine, 1 g of glycerol, and 1 g of corn starch and the mixture was stirred at 90° C. to dissolve the agar and corn starch. Then, the temperature of the resultant suspension was decreased to 70° C. Further, a solution of 1.5 g of chitosan (Chitosan LL (registered trademark), viscosity (0.5%, 20° C.); 20 cps or more, Yaizu Suisan), and 0.5 g of acetic acid in 25 ml of water was added thereto and stirred to obtain a uniform suspension.
[0118] 7 ml of the suspension was spread in a dish of 9.3 mm in inner diameter and dried to prepare a cast film (about 60 μm in film thickness).
example 3
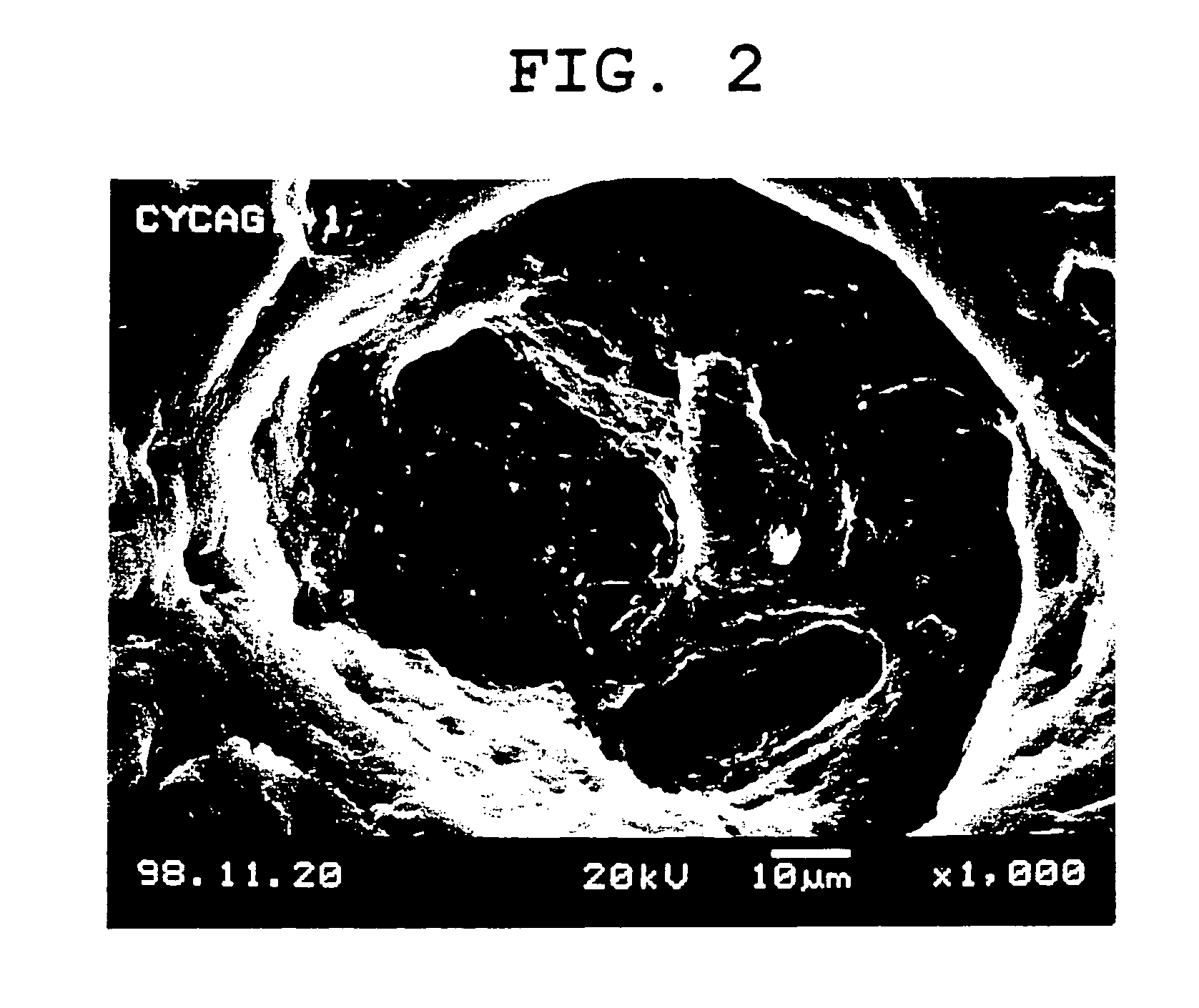
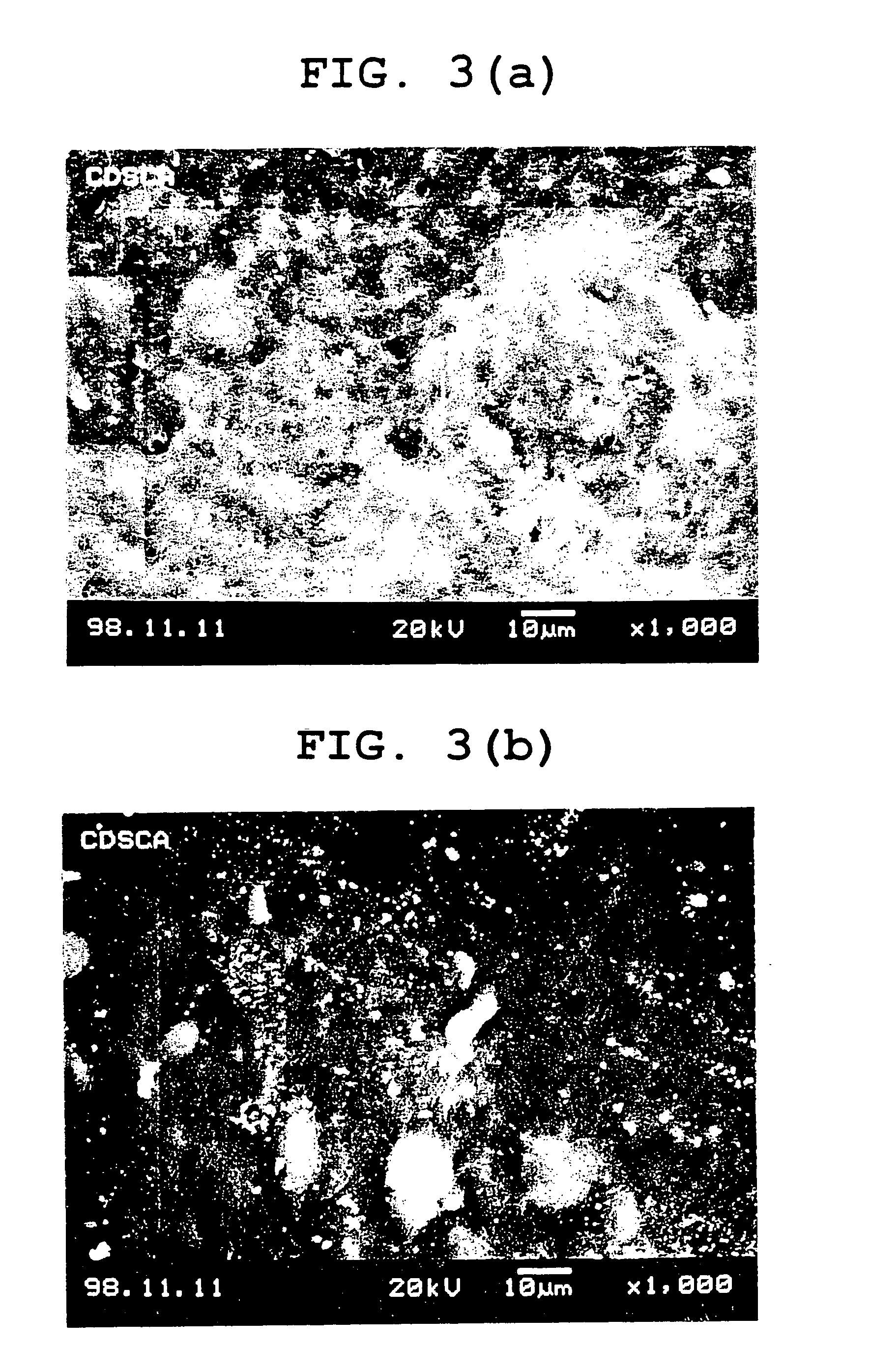
[0121] 100 ml of water was added to 2 g of agar, 8 g of cystine, and 8 g of glycerol, and the mixture was stirred at 90° C. to dissolve the agar. After the temperature of the resultant suspension was decreased to 70° C., 10 g of gelatin was added and the mixture was stirred to dissolve gelatin. Further, a solution of 6 g of chitosan and 6 g of citric acid in 60 ml of water was added thereto and stirred to obtain a uniform suspension. Then seamless soft capsules having a particle size of about 2.4 mm and a weight of about 8.9 mg (content of about 5.3 mg) were produced by a drip in oil method using the suspension as a film forming liquid and a solution (0.25 mg / g) of fat-soluble red dye Sudan IV dissolved in a medium chain fatty acid triglyceride (MCT) as a content liquid. As the chitosan, a 2.8:3.2 mixture of Chitosan LL (registered trademark) (viscosity (0.5%, 20° C.); 20 cps or more, Yaizu Suisan) and Chitosan 100 (registered trademark) (viscosity (0.5%, 20° C.); 90.2 cps, Wako) wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com