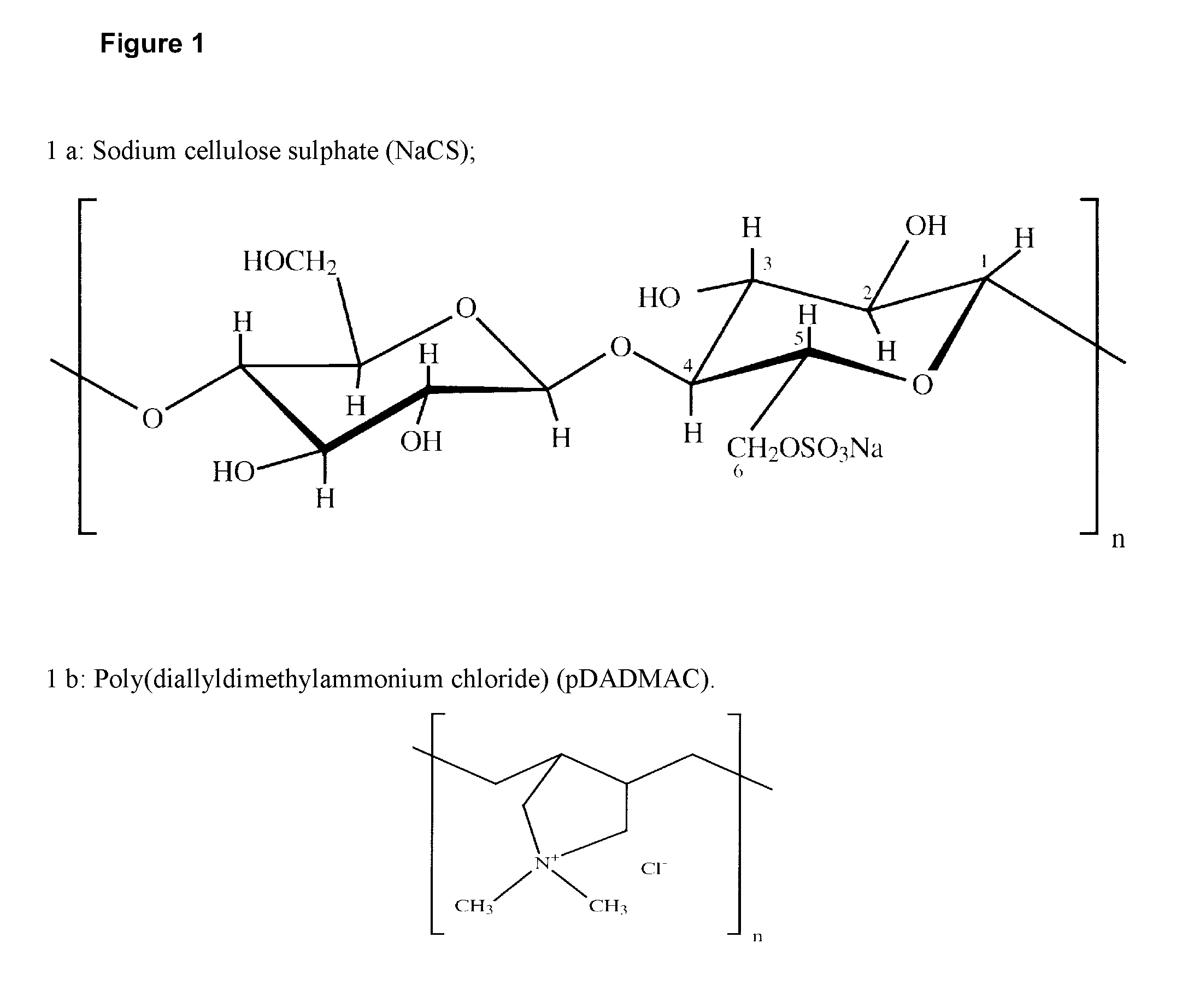
Protection of microbial cells from acidic degradation
a technology of acidic degradation and microbial cells, applied in the direction of plant ingredients, food preparations, non-active ingredients of pharmaceuticals, etc., can solve the problems of reducing the effect of microbial cells, and reducing the formation of toxins, so as to enhance the weight gain of farm animals, enhance food utilisation, and improve the effect of mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Growing of Lactobacillus acidophilus to an OD of 1.0
[0102]A culture of Lactobacillus acidophilus was started with a 20 ul sample from the thawed bacteria stock by injecting it into 50 ml MRS (named by its inventors: de Man, Rogosa and Sharpe, developed in 1960; Preparation of 1 liter of MRS medium: 51 g MRS broth powder, 1 g Polysorbate 80, 0.5 g L-cysteine hydrochloride and 999 ml of H2O adjusted to pH of 6.2.) in a 50 ml EM flask. The stock had been kept at −80° C. and was purchased from DSM (catalogue number DSM 20079) (Moro) Hansen and Mocquot (ATCC 4356). The culture was incubated overnight shaking at 50 rpm and at 37° C. On Day 1 of the experiment, the optical density of the bacterial culture was determined at 600 nm on Tecan Infinite M200. Typically the optical density at 600 nm that gives a reading of 1 will correspond to the exponential phase of the bacterial growth. The cells were grown up to an OD 600 nm reading of 1, to ensure that cells were in the exponential phase bef...
example 2
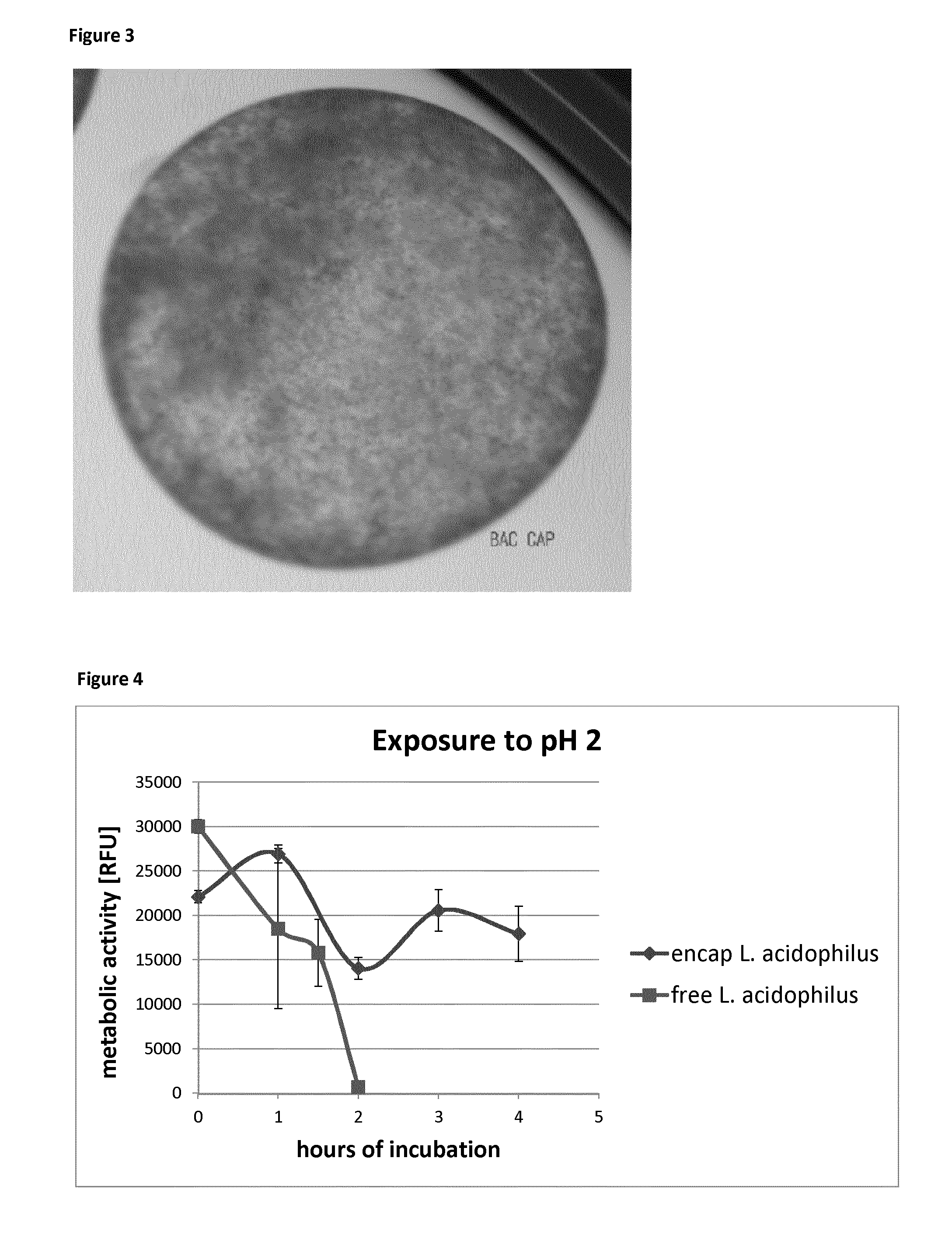
Survival of Non-Encapsulated Lactobacillus acidophilus Cells in Hydrochloric Acid
[0103]A solution of 0.01M HCl in PBS (phosphate buffered saline) was prepared by adding 4.2 ml of 37% HCl to 500 ml PBS. The pH value was adjusted to 2.0 exactly by using 5M HCl.
[0104]5 ul of the lactobacillus culture was added to 1 ml of hydrochloric acid in PBS (phosphate buffered saline salt solution) in a sterile Eppendorf tube in triplicate. As a control, 5 ul of the same Lactobacillus culture was added to 1 ml of PBS in a sterile Eppendorf tube in triplicate at 0 hr time point. The hydrochloric acid testing was carried out at different time points, i.e. after 1 hr, 1.5 hr and 2 hrs of exposure time.
[0105]At the various time points all the Eppendorf tubes were centrifuged down at speed of 3000×g for 1 min to remove hydrochloric acid. They were washed twice with MRS medium and 100 ul of MRS medium was added into the pellet. The pellet was resuspended therein and all was transferred into a 96 well pl...
example 3
Encapsulation of Lactobacillus acidophilus Cells in NaCS and pDADMAC
[0107]100 ul of the bacteria culture with an optical density of 1 were mixed with 2 ml of sodium cellulose sulphate solution containing 1.8% sodium cellulose sulphate (09-5 ul-592, provided by the Fraunhofer Institute) and 1% sodium chloride, and dropped into a 150 ml bath of 1.3% 24 kDa pDADMAC with the use of a 5 ml syringe and a 23 G needle.
[0108]The hardening time for the capsules in the pDADMAC bath was 4 mins. The capsules were then washed once for 8 min with 300 ml of 1×PBS, and once 4 mins with 300 ml of 1×PBS. These were followed by 3 washes with 30 ml 1× Phosphate Buffered Saline each and 3 washes with 30 ml MRS medium each. The capsules were then transferred to a 250 ml conical flask containing 100 ml of fresh MRS medium. These capsules were cultured at 37° C. incubator, with a speed of 50 rpm.
[0109]The AlamarBlue Assay described above was performed on the encapsulated lactobacillus cells. The assay was p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com