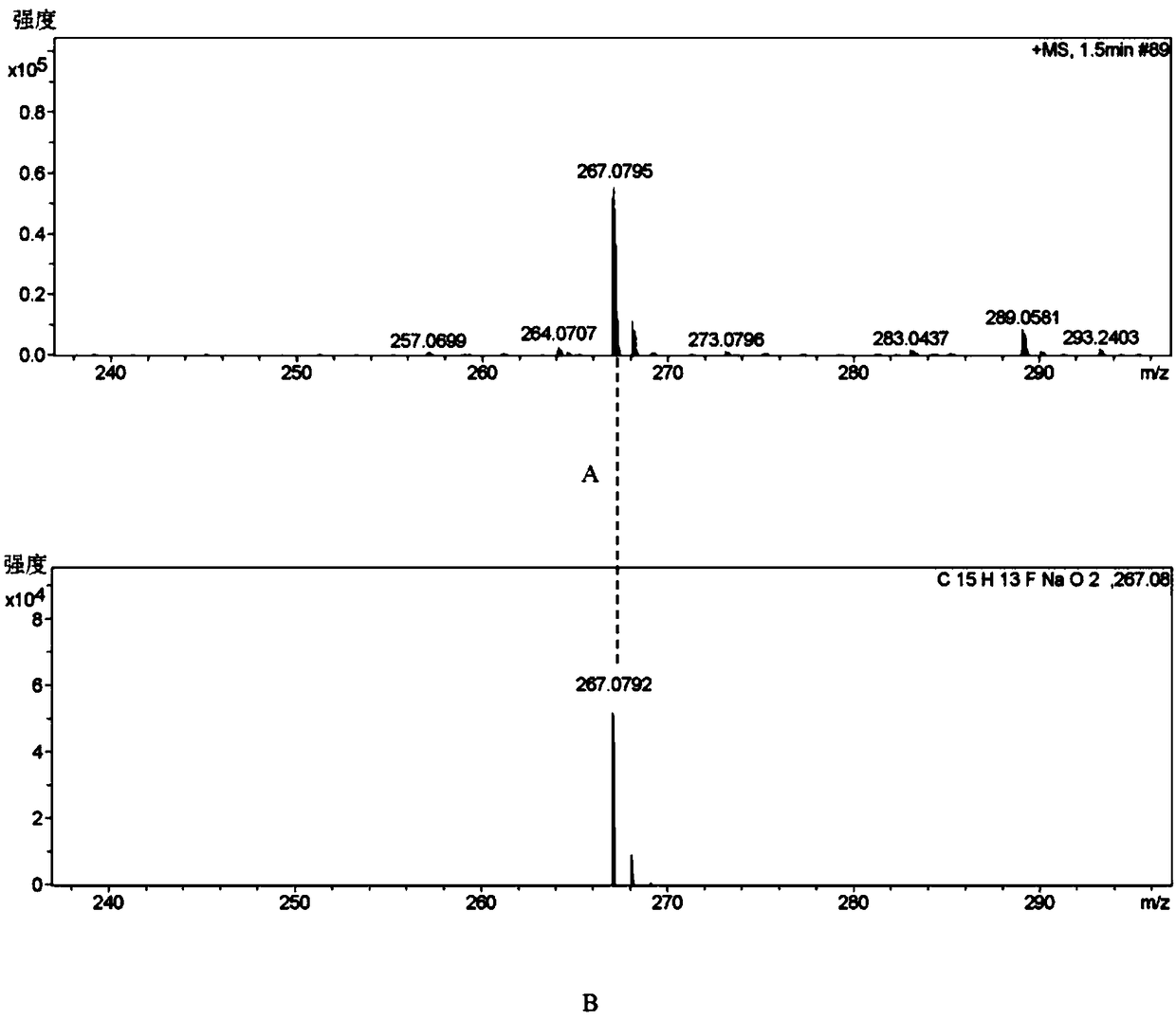
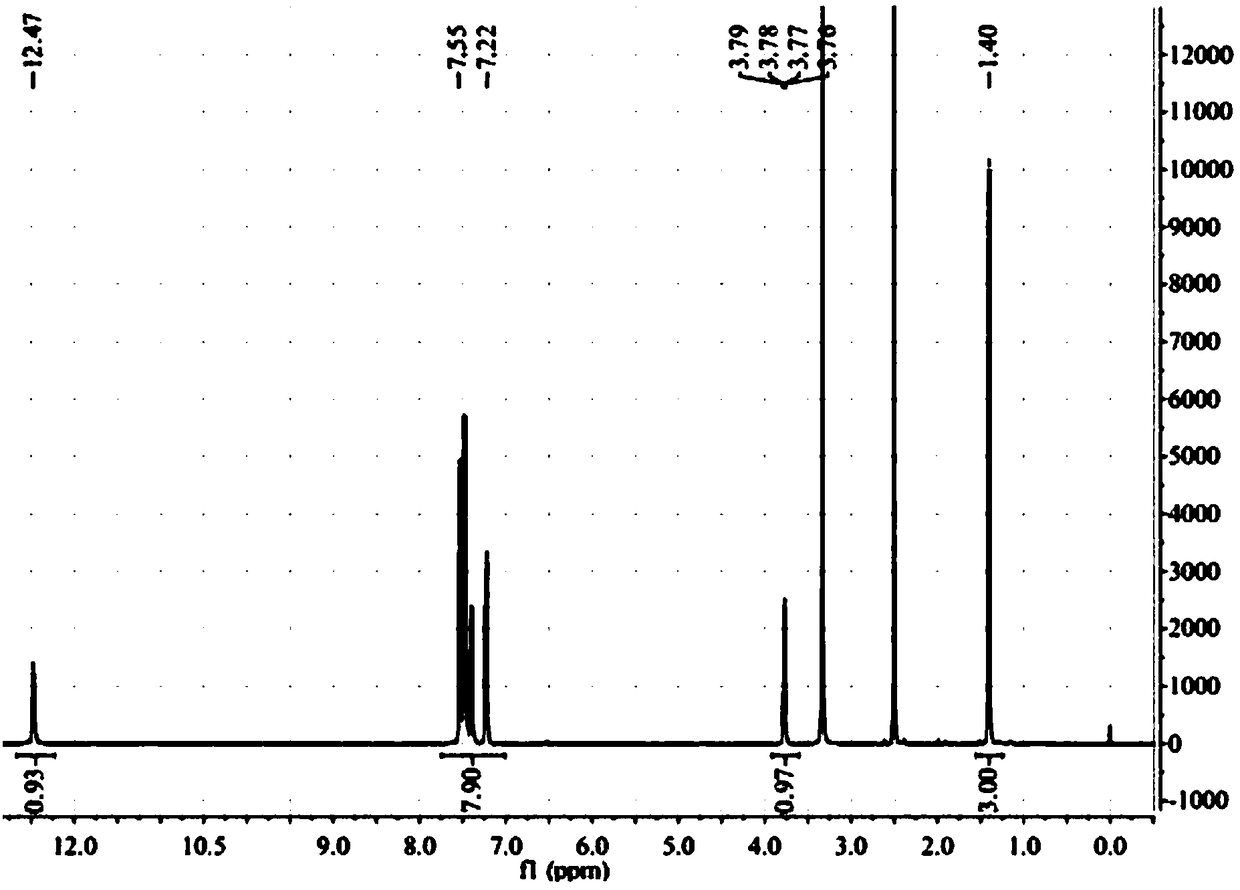
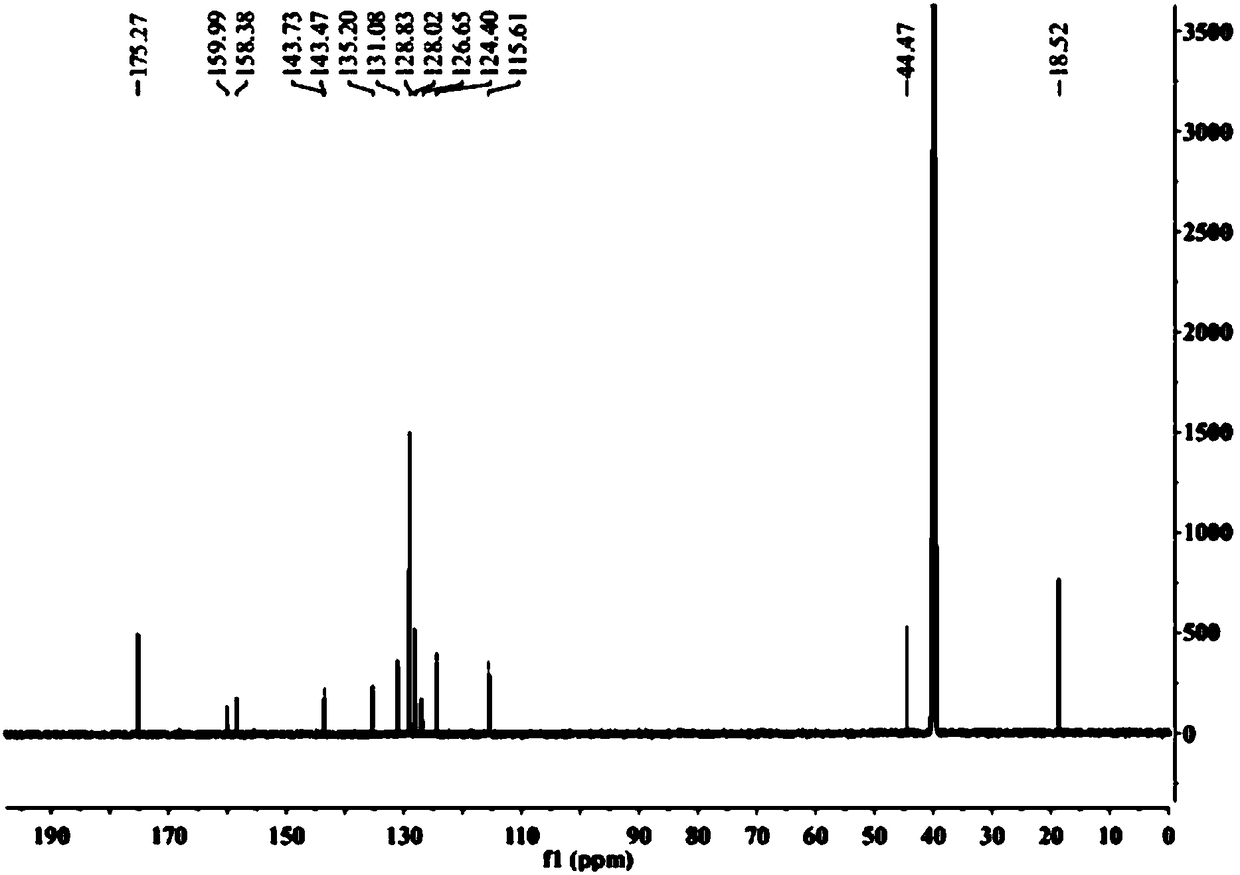
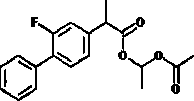
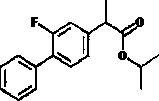
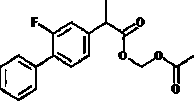
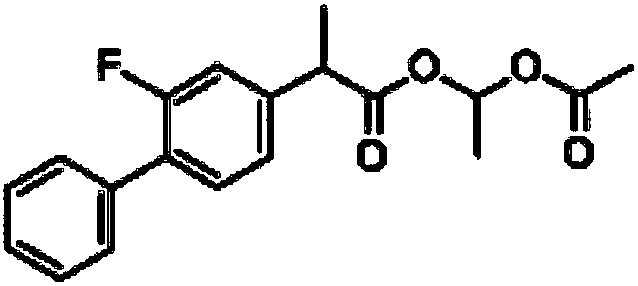
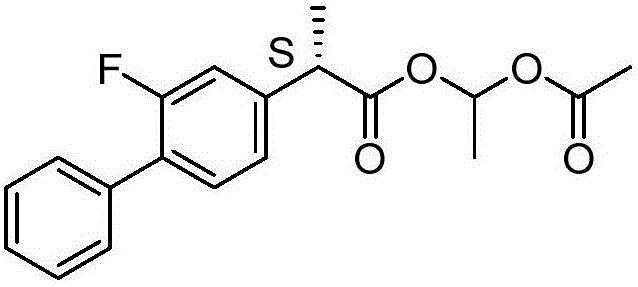
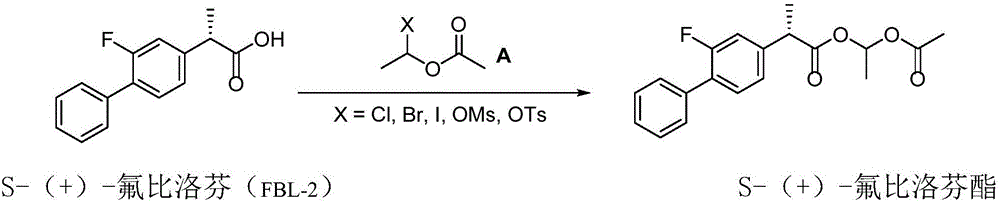
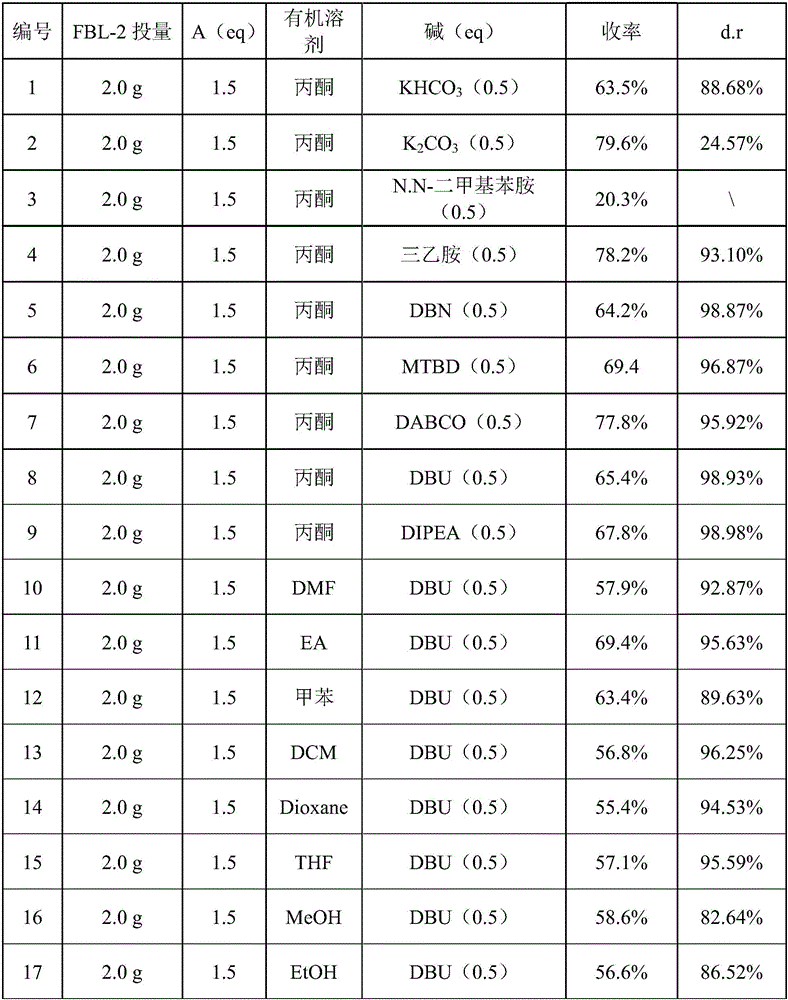
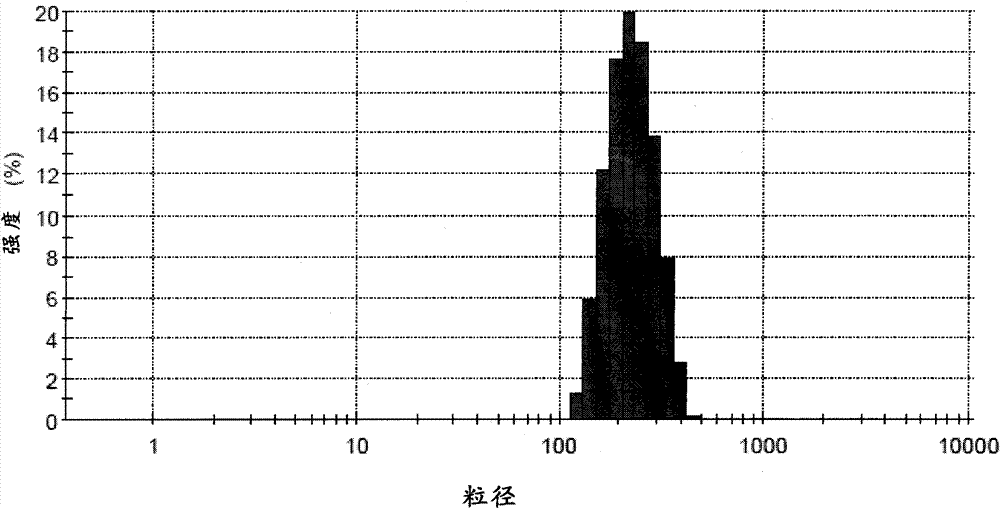
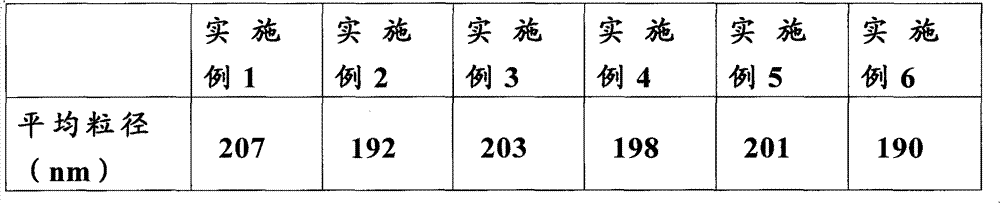
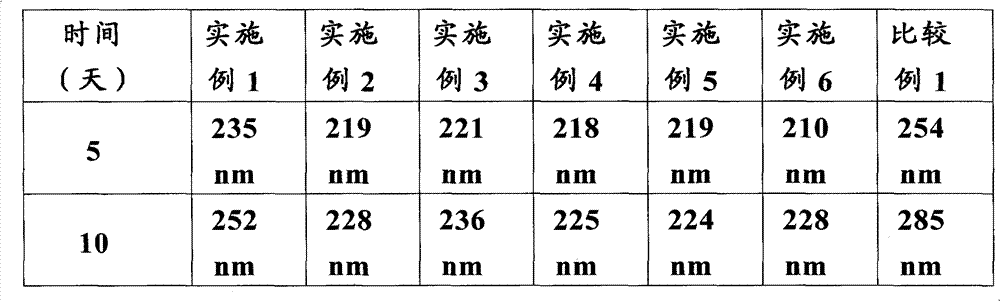
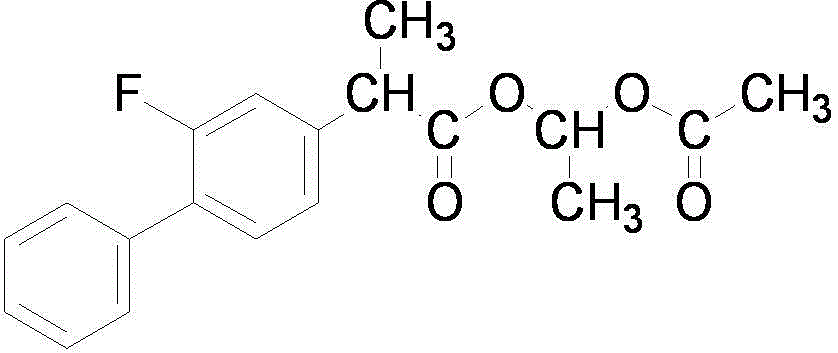
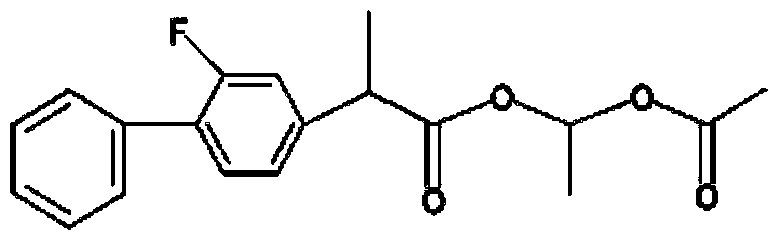
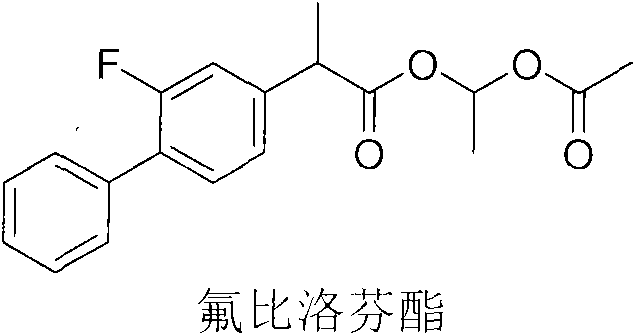
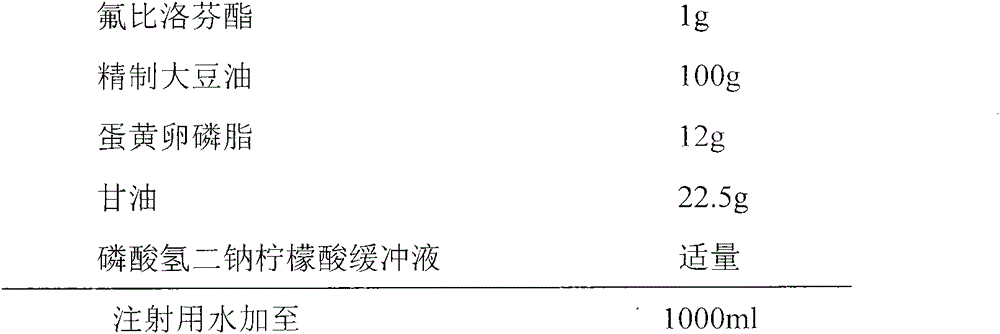
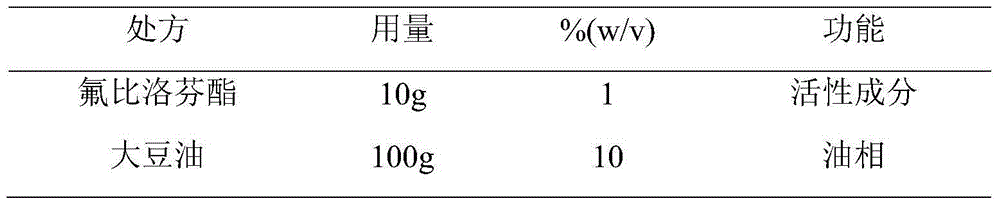
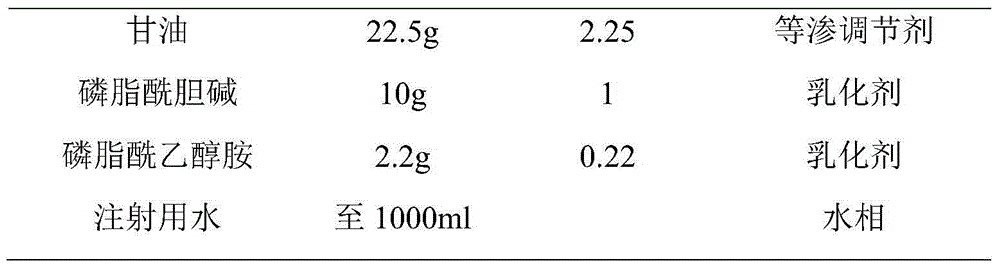
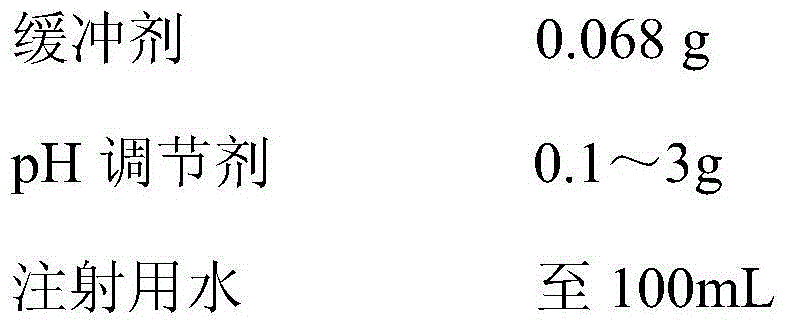Patents
Literature
79 results about "Flurbiprofen axetil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of flurbiprofen axetil
ActiveCN103012144AConvenient sourceMild reaction conditionsOrganic compound preparationCarboxylic acid esters preparationPropanoic acidDistillation
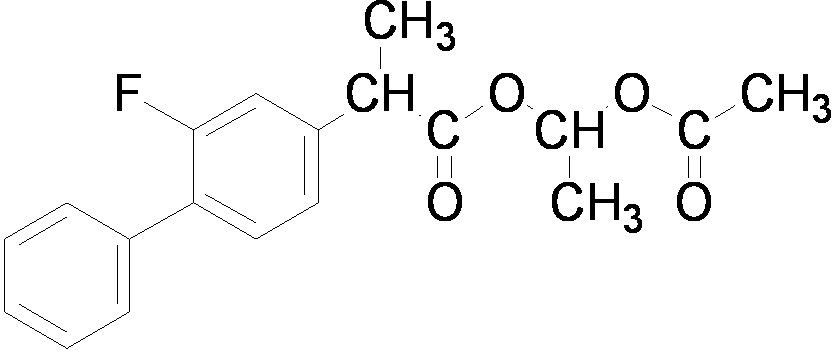
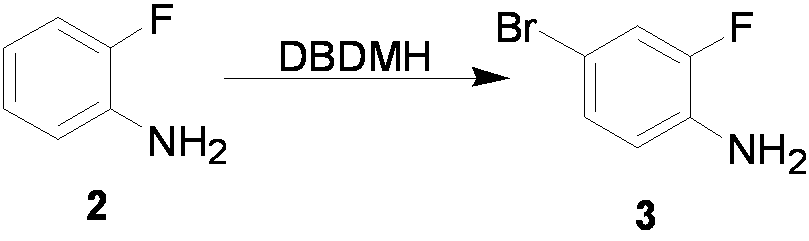
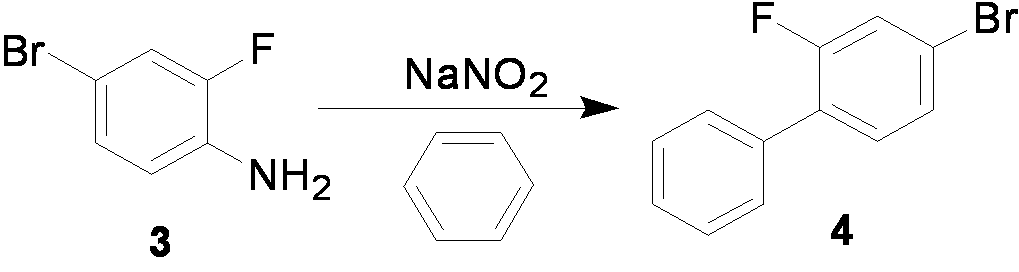
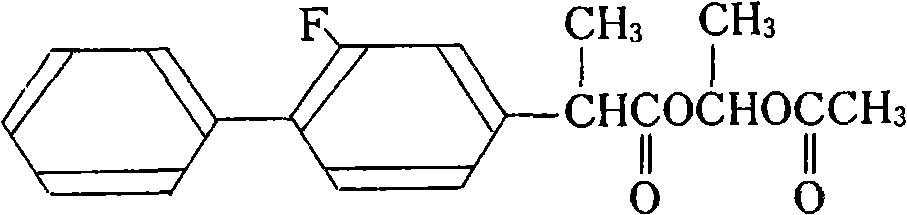
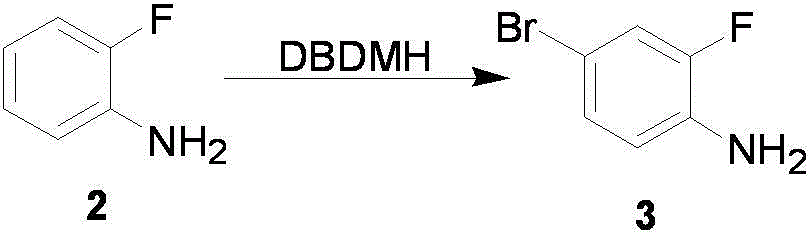
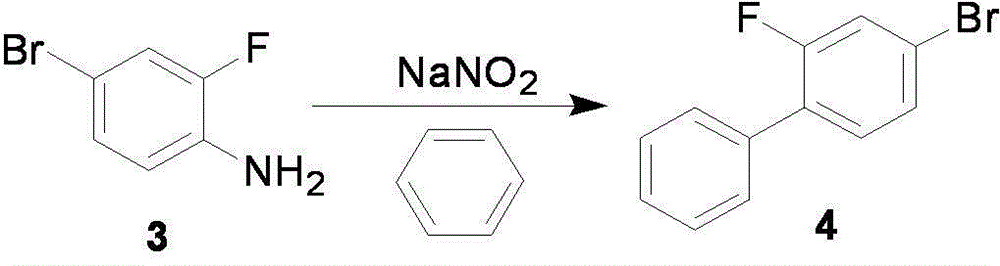
The invention relates to a preparation method of flurbiprofen axetil. The method comprises the following steps of: preparing 4-bromine-2-fluoroanili from fluoroaniline under the action of 1,3- dibromo-5,5-dimethyl hydantoin; condensing 4-bromine-2-fluoroanili with benzene under the action of a catalyst and sodium nitrite to synthesize 4-bromine-2-fluorobiphenyl; carrying out grignard reaction and acidity reaction on the 4-bromine-2-fluorobiphenyl and 2-bromine sodium propionate under the action of a catalyst to generate 2-(2- fluorine-4-biphenylyl) propionic acid; condensing the 2-(2- fluorine-4-biphenylyl) propionic acid with 1-chloroacetic ethyl acetate to generate a target compound flurbiprofen axetil; and carrying out molecular distillation on the flurbiprofen axetil crude product to obtain a flurbiprofen axetil final product.
Owner:哈药集团股份有限公司 +1
Method for preparing flurbiprofen axetil compound
ActiveCN102381970AImprove product qualityLow costOrganic compound preparationCarboxylic acid esters preparationPurification methodsN dimethylformamide
The invention discloses a method for preparing a flurbiprofen axetil compound. The method comprises the following steps: adding 1.0mol of 2-fluoro-alpha-methyl(1,1'-diphenyl)-4-acetic acid and 1L of solvent in a reaction container, stirring and heating to 70-75 DEG C, reacting for 2h, cooling to 20 DEG C, dropping 1.5-1.7mol of 1-bromoethyl acetate, reacting for 5h, and cooling; adding ethyl acetate and water, then separating out the organic phase, washing with water, washing with a saturated sodium carbonate solution, then washing with water, and finally washing with saturated salt water; and carrying out rotary evaporation on the organic phase to be dry, and vacuumizing to remove the solvent and obtain a crude product; and then dissolving the crude product with an organic solvent, adding silica gel and activated carbon, heating to reflux for 20-40min, cooling, performing suction filtering, and vacuumizing to obtain flurbiprofen axetil, wherein the solvent is N, N-dimethylformamide or potassium carbonate; and the organic solvent is a mixture of an ester solvent and an ether solvent, the volume ratio of the ester solvent to the ether solvent is 1:(5-30) and the addition amount of the organic solvent is 1-2 times that the mass of the crude product. By adopting the preparation method of the flurbiprofen axetil compound, the problems of the existing purification method can be well solved; the method has low cost, simpleness in operations and good product quality; and the quality of the product obtained through the experiment is higher than the import standard.
Owner:NANJING YOKO PHARMA GRP CO LTD
Flurbiprofen axetil medium-chain and long-chain fat emulsion and preparation method thereof
InactiveCN101940549AEasy to shapeSolve the real problemOrganic active ingredientsAntipyreticFat emulsionsFlurbiprofen axetil
The invention relates to a flurbiprofen axetil medium-chain and long-chain fat emulsion and a preparation method thereof. The fat emulsion comprises flurbiprofen axetil, long-chain oil for injection, medium-chain oil for injection, emulsifier, isotonic agent, pH regulator and water for injection, wherein the weight-volume percentage of the flurbiprofen axetil is 0.5-5%(w / v), the weight-volume percentage of the long-chain oil for injection is 5-10%(w / v), the weight-volume percentage of the medium-chain oil for injection is 5-10%(w / v), and the weight-volume percentage of the isotonic agent is 1-5%(w / v).
Owner:北京中海康医药科技发展有限公司
Preparation method of flurbiprofen and preparation method of flurbiprofen axetil
InactiveCN108558651ALow costHigh purityPreparation from carboxylic acid saltsOxygen-containing compound preparationChemical synthesisBromine
The invention relates to the field of pharmaceutical chemical synthesis, in particular to a preparation method of flurbiprofen and a preparation method of flurbiprofen axetil. The preparation method of the flurbiprofen comprises the steps of carrying out a Grignard reaction by using 4-bromine-2-fluorine biphenyl as a raw material, carrying out a coupling reaction, and acidizing to obtain the flurbiprofen; the yield is 90%, and the purity is 99.5%; then, the flurbiprofen axetil is prepared by using the flurbiprofen, obtained by the method, as a raw material, the yield reaches up to 90%, and thepurity reaches up to 99.5%. The preparation methods are high in quality controllability and industrial reproducibility.
Owner:上海峰林生物科技有限公司
Flurbiprofen axetil microsphere preparation
The invention provides a flurbiprofen axetil microsphere preparation, a preparation method and an application thereof. The plurbiprofen axetil microsphere preparation comprises flurbiprofen axetil, an oil phase solvent and chitosan and / or derivatives thereof and an emulsifying agent. The method for preparing the flurbiprofen axetil microsphere preparation comprises the following steps of: (1) mixing oil phase mixture containing the flurbiprofen axetil, the oil phase solvent and the emulsifying agent so as to produce a uniform oil phase; (2) mixing aqueous phase mixture containing chitosan and / or the derivatives thereof and water so as to form a uniform aqueous phase; (3) adding the oil phase into the aqueous phase so as to form initial emulsion; and (4) homogenizing the initial emulsion. The invention further provides an application of the flurbiprofen axetil microsphere preparation to be used for preparing analgesic or anti-inflammatory drugs.
Owner:WUHAN DOCAN PHARMA
Flurbiprofen axetil eye nano-emulsion in-situ gel preparation and preparation method thereof
InactiveCN101385697AIncrease system viscosityNon-irritatingOrganic active ingredientsSenses disorderGel preparationPolyoxyethylene castor oil
The invention relates to an ophthalmic flurbiprofen ester nano emulsion-in situ gel preparation and a preparation method thereof. The ophthalmic flurbiprofen ester nano-emulsion in situ gel preparation is made from oil, an emulsifier, a thickener, an osmoregulator, a bacteriostatic agent and purified water; and is characterized in that one or a plurality of safe and non-irritating lecithin, Tween60, Tween80, polyoxyethylene castor oil and polyoxyethylene hydrogenated castor oil are taken as the emulsifier; and a high molecular material with ion sensitivity characteristic is taken as the thickener. The ophthalmic flurbiprofen ester nano emulsion-in situ gel preparation is low-viscosity fluid with good fluidity in vitro, and rapidly forms hydrogel after being dropped in eyes. The ophthalmic flurbiprofen ester nano emulsion in-situ gel preparation has the advantages of increasing the residence time of the drug in eyes and increasing bioavailability, has no irritability or other toxic side effect to eyes, and has good biocompatibility and the like.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Preparation method of flurbiprofen axetil
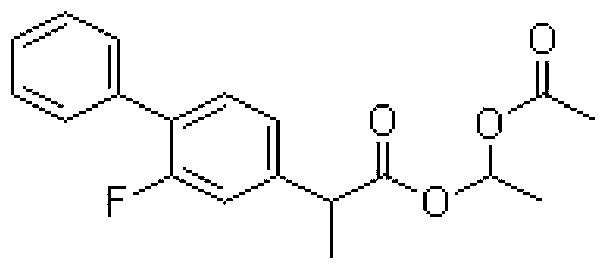
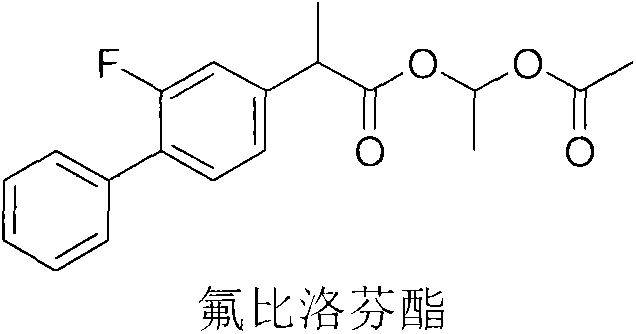
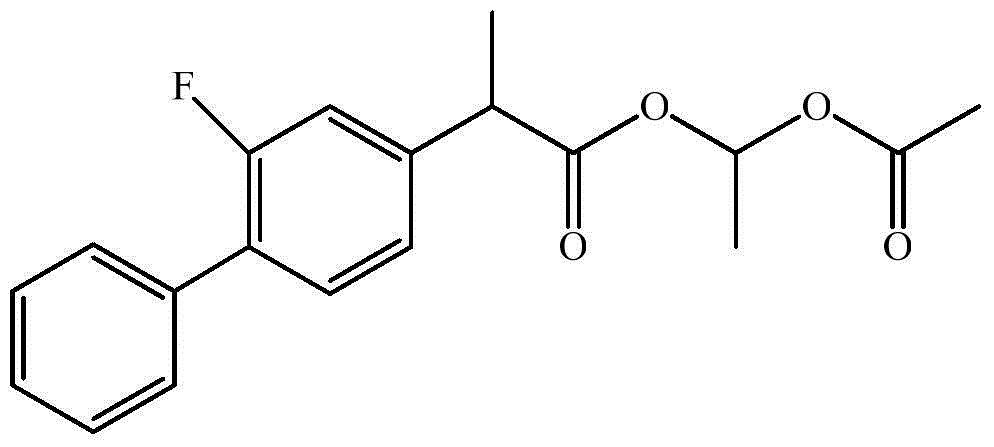
The invention relates to a preparation method of flurbiprofen axetil. The method comprises the following steps: carrying out a reaction between flurbiprofen and bromoethyl acetate in the presence of a catalyst and a solvent; washing reaction products and separating grease; carrying out underpressure distillation; dissolving a fraction in an organic solvent, washing and drying; and removing the solvent. Purity of flurbiprofen axetil prepared by the method can reach more than 99.0%.
Owner:WUHAN DOCAN PHARMA
Middle/long chain triglyceride flurbiprofen axetil injection and preparation method thereof
InactiveCN102552133AApplicable useTo achieve the therapeutic effect of anti-inflammatory and analgesicOrganic active ingredientsAntipyreticCritically illSide effect
The invention provides anti-inflammatory and analgesic flurbiprofen axetil injection and a preparation method thereof. A mixture of 0.1-10 percent (w / v) of flurbiprofen axetil, 10-20 percent (w / v) of middle chain triglyceride (MCT) and 10-20 percent (w / v) of long chain triglyceride (LCT) is contained in the prescription (wherein the proportion of MCT and LCT is 4:1-1:4). The preparation method comprises the following steps: taking 1-5 percent (w / v) of lecithin as an emulsifying agent, taking 1-5 percent (w / v) of glycerol as an isotonic agent, finally adding disodium hydrogen phosphate, citric acid and water for injection and adjusting the pH value to 4-7 to prepare flurbiprofen axetil fat emulsion injection through colostrums, homogeneity and sterilization. The injection has the beneficial effects that the anti-inflammatory and analgesic effect is taken more quickly; the accumulation of fat of a human body can be reduced, and fatty tissues and liver load can be reduced; and the injection is more suitable for critically ill patients and people with poor liver functions and has lower toxic and side effects.
Owner:GUANGDONG JIABO PHARM CO LTD
Application of (R)- flurbiprofenester in pharmacy, injection thereof and method for preparing the same
The invention relates to the use of (R)- flurbiprofenester in preparing medicament for analgesia and treatment of cancer and senile dementia, the invention also relates to a (R)-Flurbiprofen Axetil injection as main ingredient of the medicament, emulsifier of emulsibility constituents and solvent of soluble constituents. The invention also relates to the process for preparing the (R)-Flurbiprofen Axetil injection.
Owner:黄文豪
Flurbiprofen axetil fat emulsion concentrate and preparation method and use thereof
InactiveCN104706575ANo delaminationImprove liquidityOrganic active ingredientsAntipyreticPharmacyFat emulsions
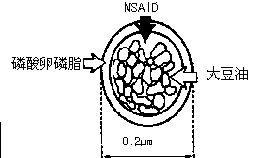
The invention relates to a flurbiprofen axetil fat emulsion concentrate and a preparation method and use thereof, and belongs to the field of medicine and pharmacy. The flurbiprofen axetil fat emulsion concentrate overcomes the defects of complex traditional fat milk preparation process and poor product stability. The flurbiprofen axetil fat emulsion concentrate is simple in preparation process only needing simple physical mixing without homogenization process. The product can be sterilized through a 0.22 mum millipore filter film, can spontaneously emulsify during clinical use by dilution with normal saline or glucose solution and other water solutions and slight oscillation, under the optimal conditions, emulsified average particle size is about 0.2 mum, and fully shows injection fat milk properties. The flurbiprofen axetil fat emulsion concentrate product has good liquidity, and may not be hung on the wall, the appearance is single-phase, transparent and clear, clarity detection is acceptable, and after repeated freezing and thawing, preparation stratification phenomenon does not occur. The flurbiprofen axetil fat emulsion concentrate product is used for postoperative and cancer analgesia and other clinical application.
Owner:天津迈迪瑞康生物医药科技有限公司
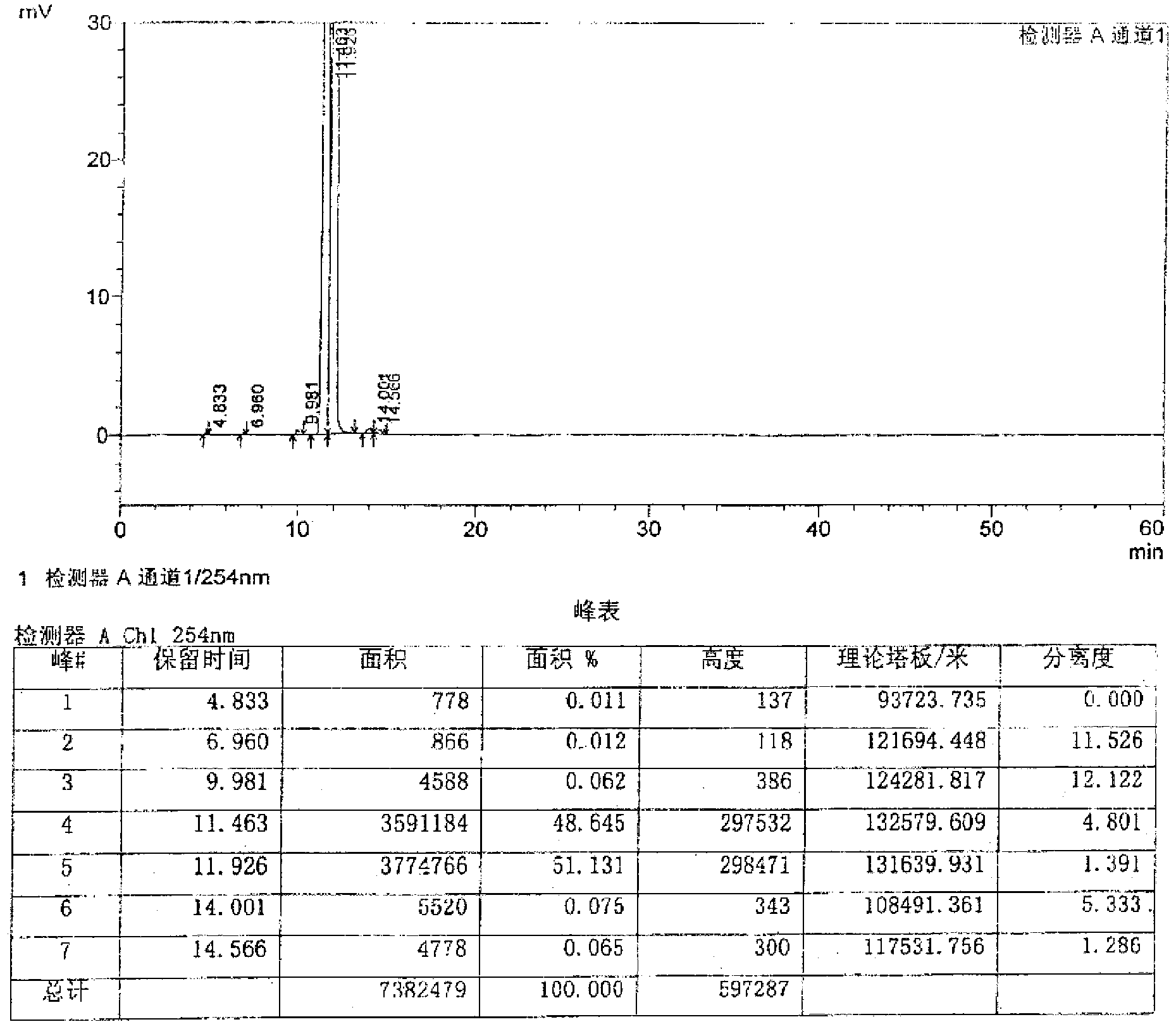
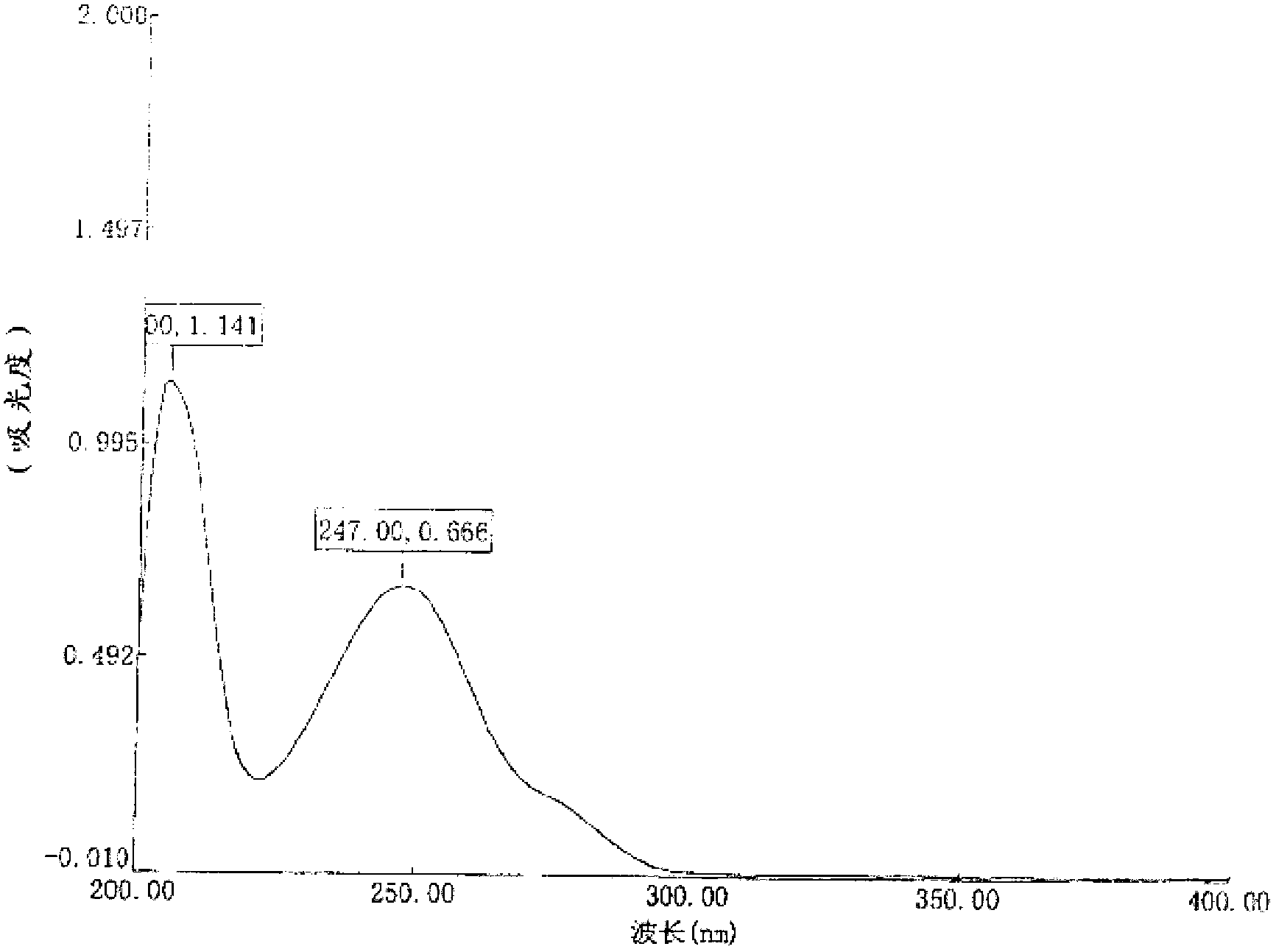
Detection method of related substance in flurbiprofen axetil injection
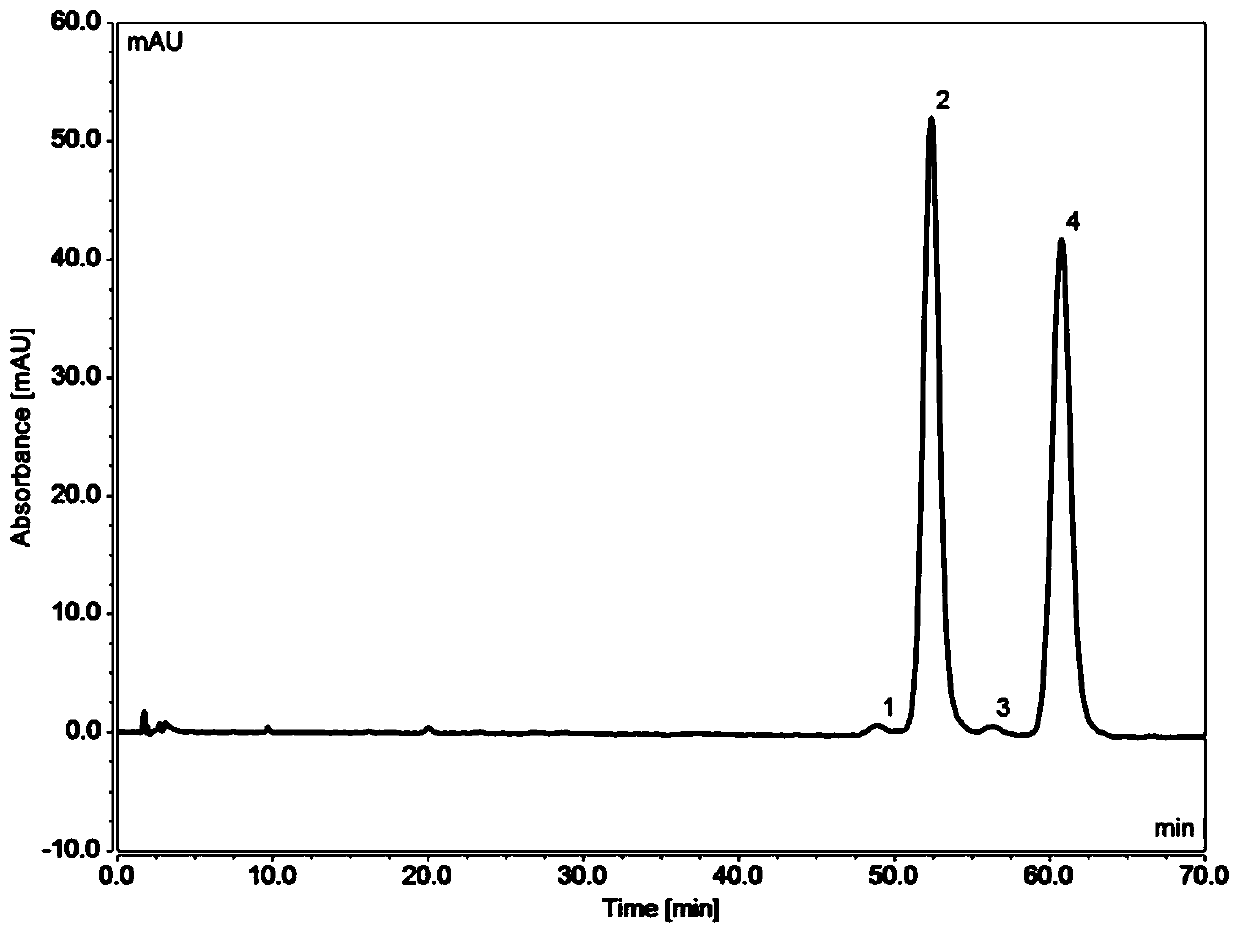
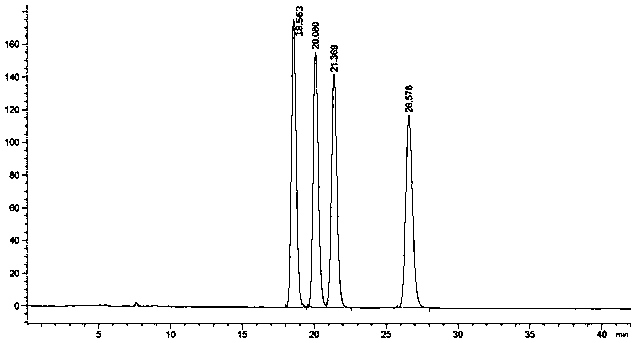
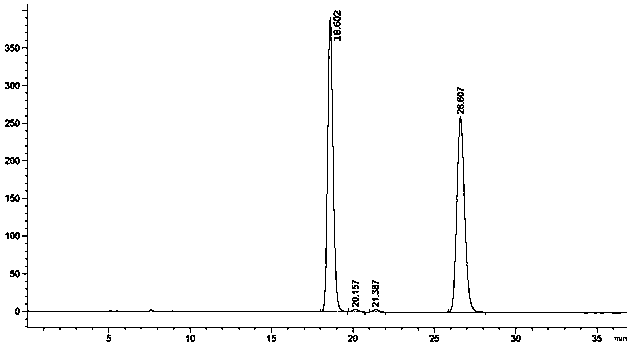
The invention provides a detection method of a related substance in flurbiprofen axetil injection. Two pairs of enantiomers and related substances thereof of the flurbiprofen axetil in the flurbiprofen axetil injection can be well separated by use of a reverse high performance liquid chromatography method after adopting gradient elution.
Owner:天津蓝丹企业管理咨询合伙企业(有限合伙)
Liquid chromatography separation and detection method of flurbiprofen axetil enantiomer and impurity A
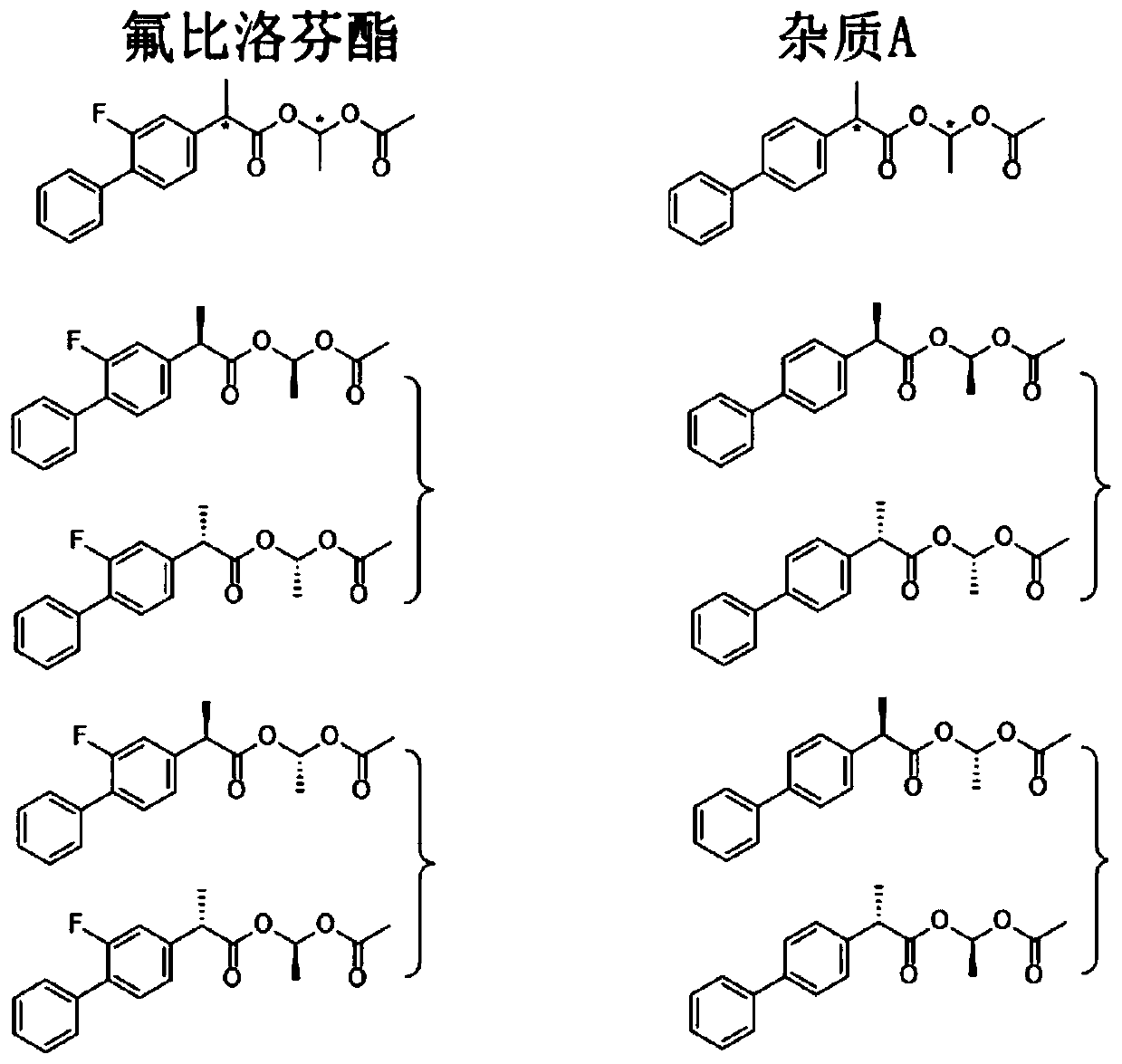
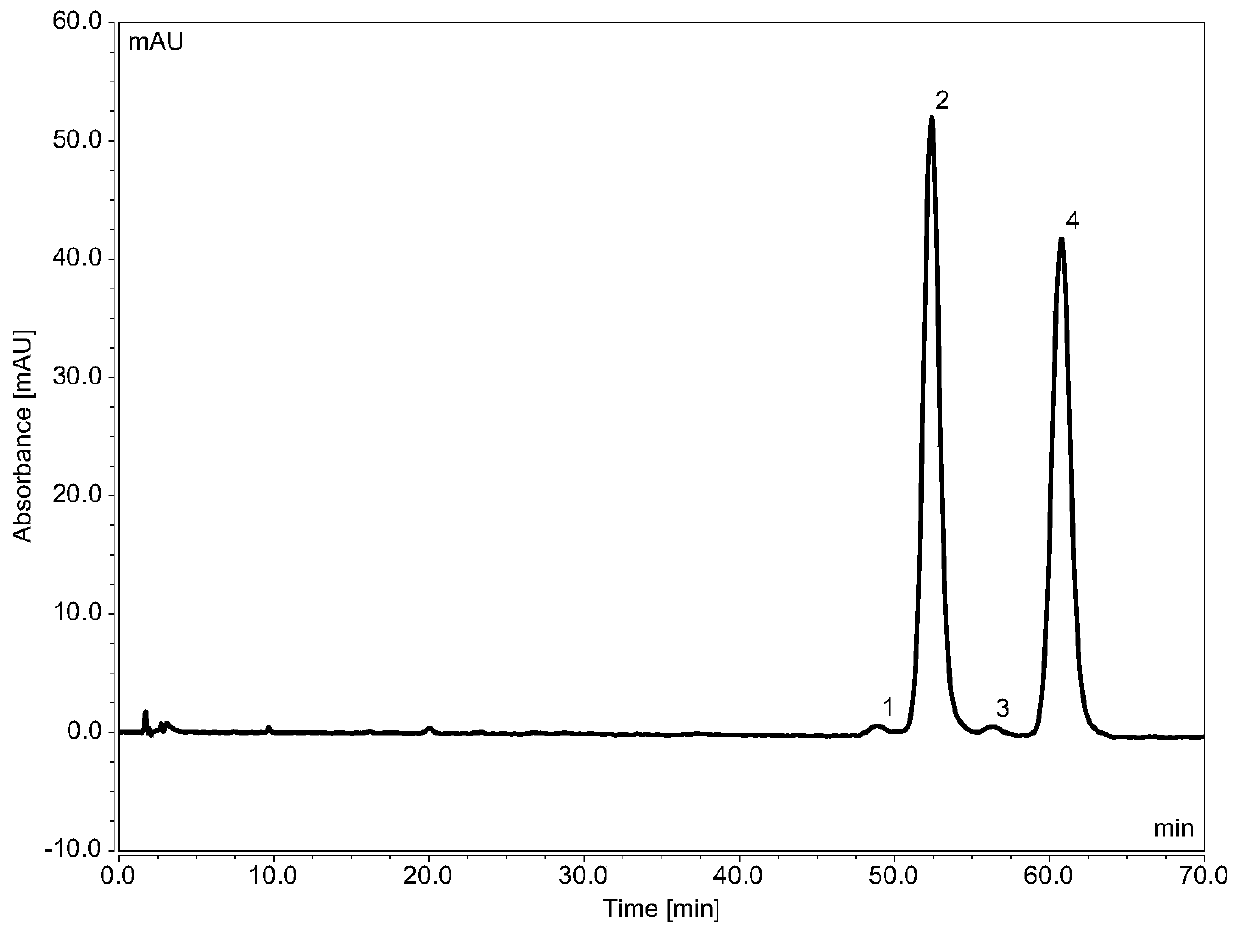
ActiveCN110988230ALittle effect of fillerAchieve Separation ResultsComponent separationO-Phosphoric AcidEnantiomer
The invention provides a liquid chromatography separation and detection method of flurbiprofen axetil enantiomer and impurity A. The method comprises the following steps: diluting and dissolving flurbiprofen axetil feed liquid to be detected and impurity A control liquid; loading a flurbiprofen axetil test solution containing an impurity A onto an octadecylsilane chemically bonded silica column and a pentafluorophenylsilane chemically bonded silica series column, and carrying out separation detection on flurbiprofen axetil; carrying out isocratic elution by taking methanol and a phosphoric acid aqueous solution as mobile phases; and after the elution is finished, detecting by using an ultraviolet detector to obtain the signal intensity of the sample to be detected, substituting the obtained signal intensity into the corresponding standard curve, and calculating to obtain the concentrations of flurbiprofen axetil and enantiomer thereof, and the impurity A and enantiomer thereof in the sample to be detected. The enantiomer of flurbiprofen axetil and the enantiomer of the impurity A can be well separated, the separation degree is good, the separation result is stable, and the reproducibility and stability of the method are excellent.
Owner:纳谱分析技术(苏州)有限公司
Novel 2-(2-fluorine-4biphenyl)-propionic acid pharmaceutical composition
ActiveCN103301101AReduce gastrointestinal adverse reactionsIndustrial applicabilityOrganic active ingredientsNervous disorderArgininePropionine
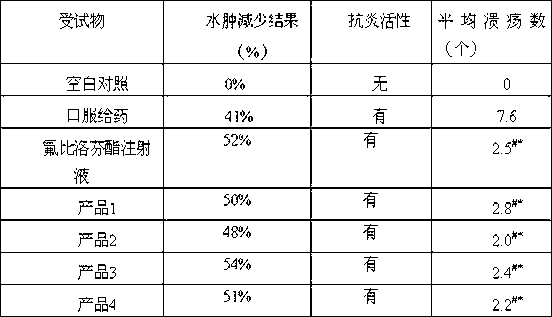
The invention provides a novel 2-(2-fluorine-4biphenyl)-propionic acid pharmaceutical composition. Flurbiprofen and basic amino acid arginine or lysine form a pharmaceutical composition solution. The composition can be administrated in an injection or oral administration manner and can also be further administrated in a freeze-drying manner. Compared with a flurbiprofen axetil injection, a flurbiprofen composition not only does not influence the antipyretic, anti-inflammation and analgestic effects of the flurbiprofen, but also has the advantages of simple technology, low cost, high quality, stable long shelf life and convenience in quality control, storage and transportation; moreover, compared with a conventional oral preparation, the flurbiprofen composition has the same low gastrointestinal tract adverse reaction as the flurbiprofen axetil injection.
Owner:南京星福星医药科技有限公司
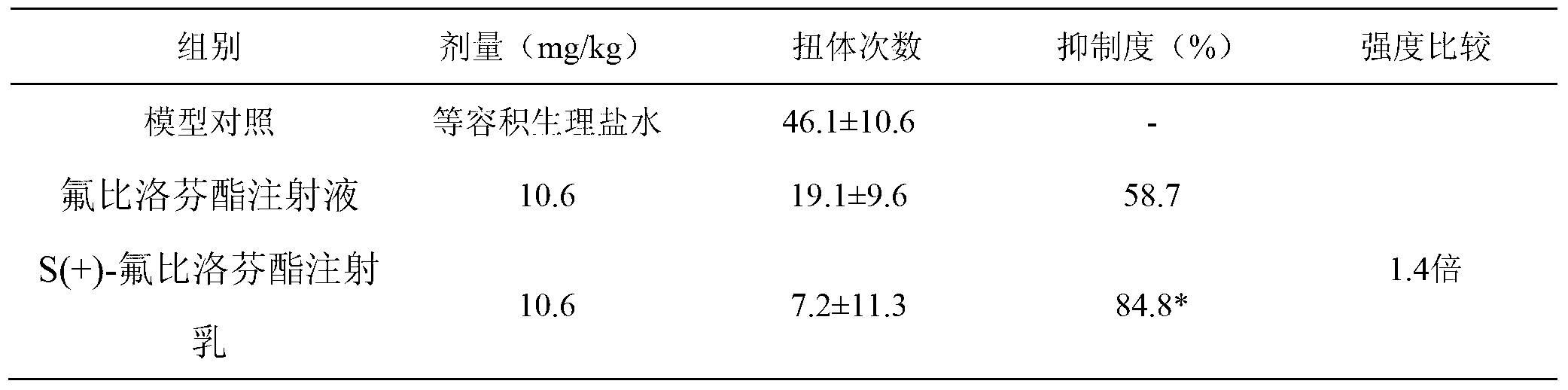
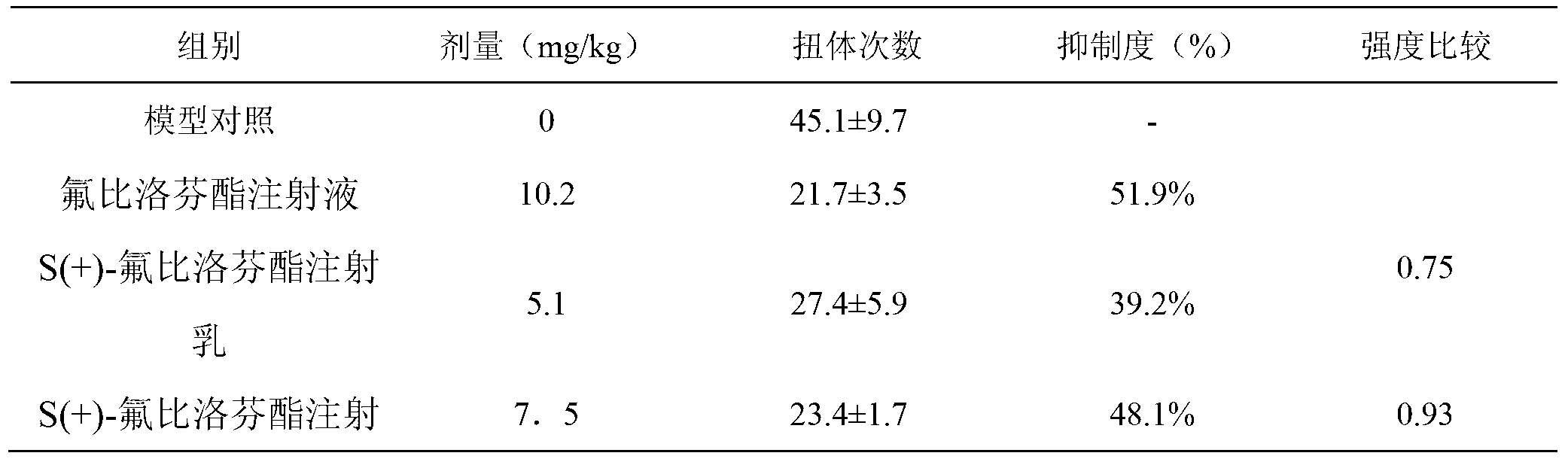
S (+) -flurbiprofen axetil emulsion for injection
The invention discloses application of a low-dosage esflurbiprofen axetil emulsion for injection. For the S (+) -flurbiprofen axetil emulsion for injection provided by the invention, when a certain curative effect is realized, the needed dosage of the S (+) -flurbiprofen axetil emulsion for injection is only 25%-75% of the dosage of the market flurbiprofen axetil emulsion, correspondingly, the clinic treatment dosage of the S (+) -flurbiprofen axetil emulsion for injection is 25%-75% of the market flurbiprofen axetil emulsion.
Owner:FUKANGREN BIO PHARMA
Flurbiprofen axetil fat emulsion injection
The invention relates to flurbiprofen axetil fat emulsion injection which comprises flurbiprofen axetil, oil and egg yolk lecithin, wherein the concentration of the flurbiprofen axetil is 0.1-1.0mg / ml. The flurbiprofen axetil fat emulsion injection prepared by the invention can be used for fever abatement of yeast-induced febrile rats.
Owner:BEIJING LANDAN PHARMA TECH
Preparation methods of flurbiprofen axetil compound
ActiveCN103664606AEasy to getLow costOrganic compound preparationCarboxylic acid esters preparationAcetic acidHigh volume manufacturing
The invention discloses preparation methods of a flurbiprofen axetil compound. The methods comprise the steps: firstly dropwise adding an ester compound into a mixture of flurbiprofen, an acid catalyst and an organic solvent to form a reaction system, or directly mixing flurbiprofen, an alcohol and the acid catalyst, after a reaction is finished, thus obtaining a reaction system, then carrying out spin steaming concentration treatment under reduced pressure, adding ethyl acetate and water to fully dissolve, standing and layering to obtain an organic phase, washing the organic phase, drying, purifying by passing through a silica gel column, and thus obtaining the flurbiprofen axetil compound. The preparation methods have the advantages of simple reaction operation, low requirements on production personnel and production equipment, small pollution degree and low production cost, are safe and effective, can avoid use of high-risk and high-toxic materials, and can be used for mass production.
Owner:GUANGDONG JIABO PHARM CO LTD
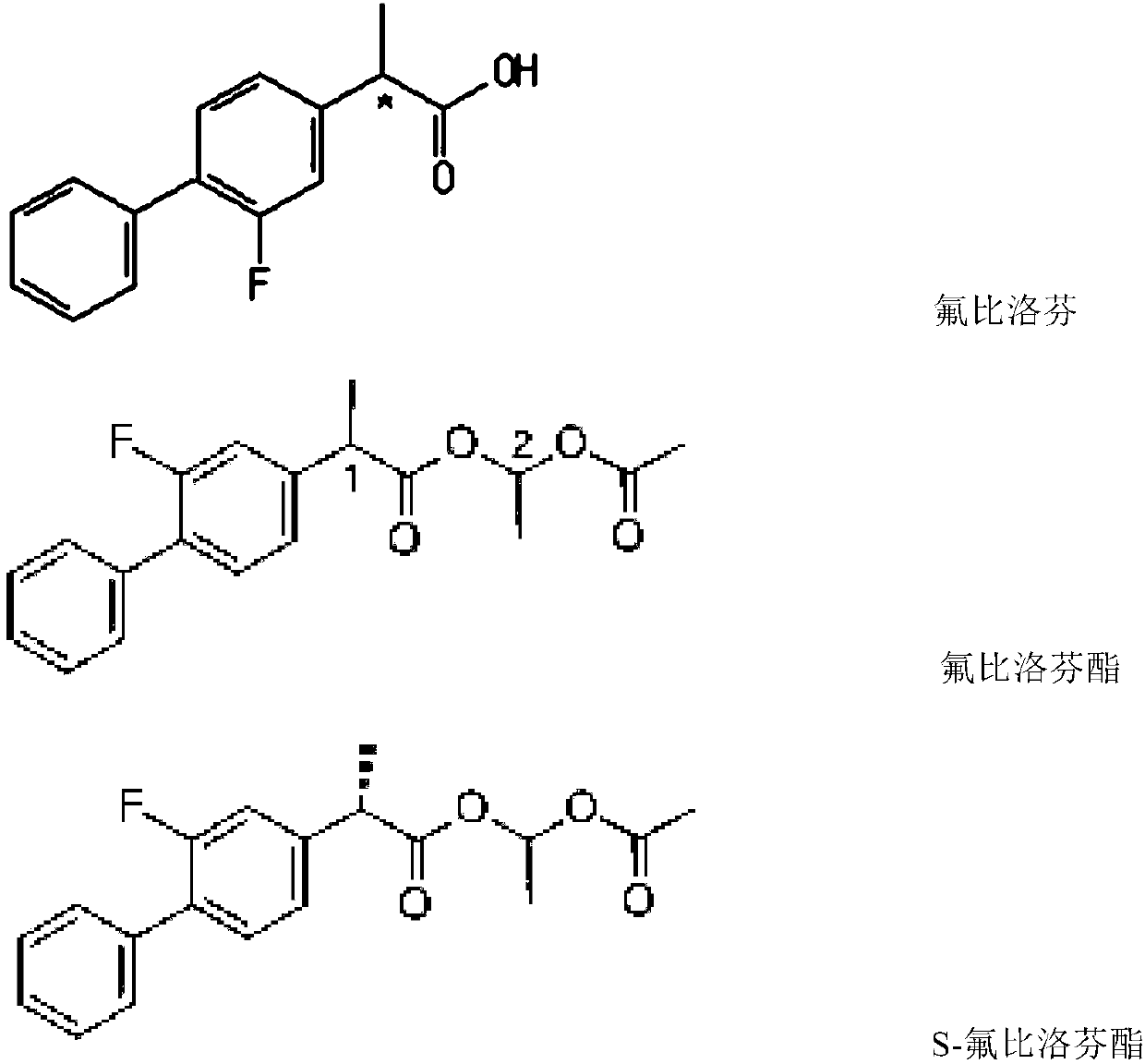
Method for resolving and determining flurbiprofen axetil and S-flurbiprofen axetil
The invention relates to a method for resolving and determining flurbiprofen axetil and S-flurbiprofen axetil, in particular to a method for resolving and determining flurbiprofen axetil and S-flurbiprofen axetil by using an HPLC method. The method adopts a starch column to perform resolution separation analysis for various products of the flurbiprofen axetil and S-flurbiprofen axetil, and the excellent chromatography effect in the description of the invention can be presented.
Owner:SHANGHAI WHITTLONG PHARMA INST +1
Flurbiprofen axetil micro-emulsion gel preparation and preparation method thereof
InactiveCN102920651AImprove efficacyImprove efficiencyOrganic active ingredientsAntipyreticEmulsionMedicine
The invention discloses a flurbiprofen axetil micro-emulsion gel preparation and a preparation method thereof. The flurbiprofen axetil micro-emulsion gel preparation is characterized in that the preparation is composed of a gel substrate and a therapeutically effective amount of medicine-containing micro-emulsion loaded on the substrate. The preparation method provided by the invention has the advantages of simple process and easy operation. The prepared micro-emulsion gel preparation can be prepared into patches, such that a novel administration mode of flurbiprofen axetil is provided, and flurbiprofen axetil efficacy and application efficiency are improved.
Owner:SHANGHAI UNIV OF ENG SCI
Flurbiprofen axetil emulsion for injection and preparation method thereof
ActiveCN109223712AImprove stabilityGood emulsifying effectOrganic active ingredientsAntipyreticMedicinePharmaceutical drug
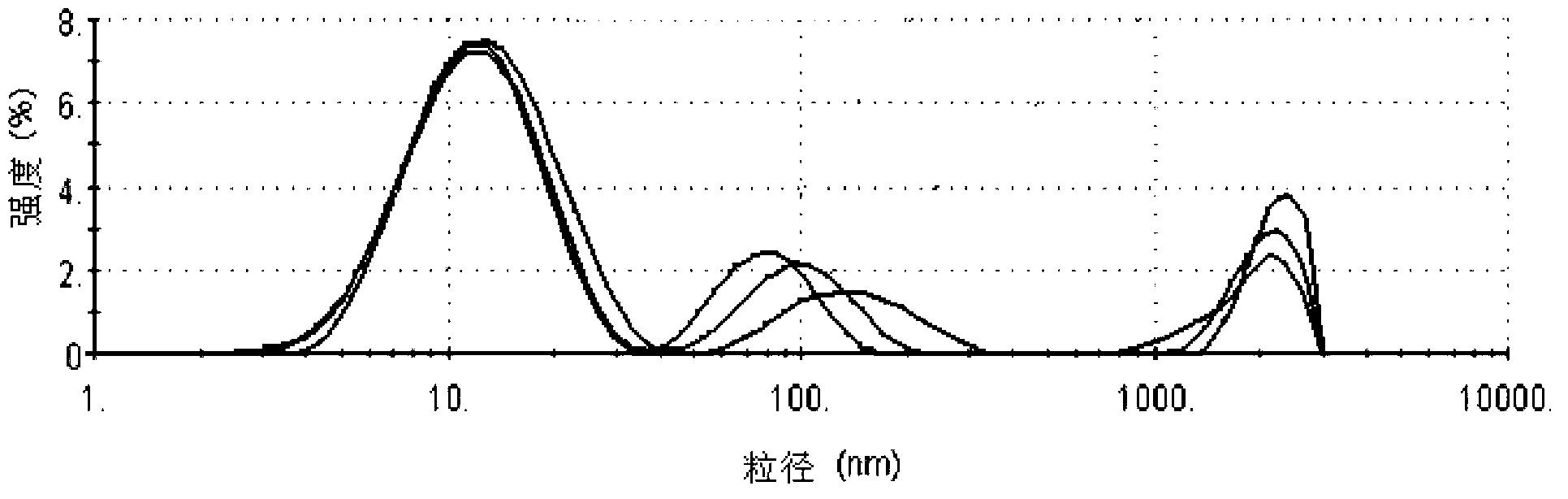
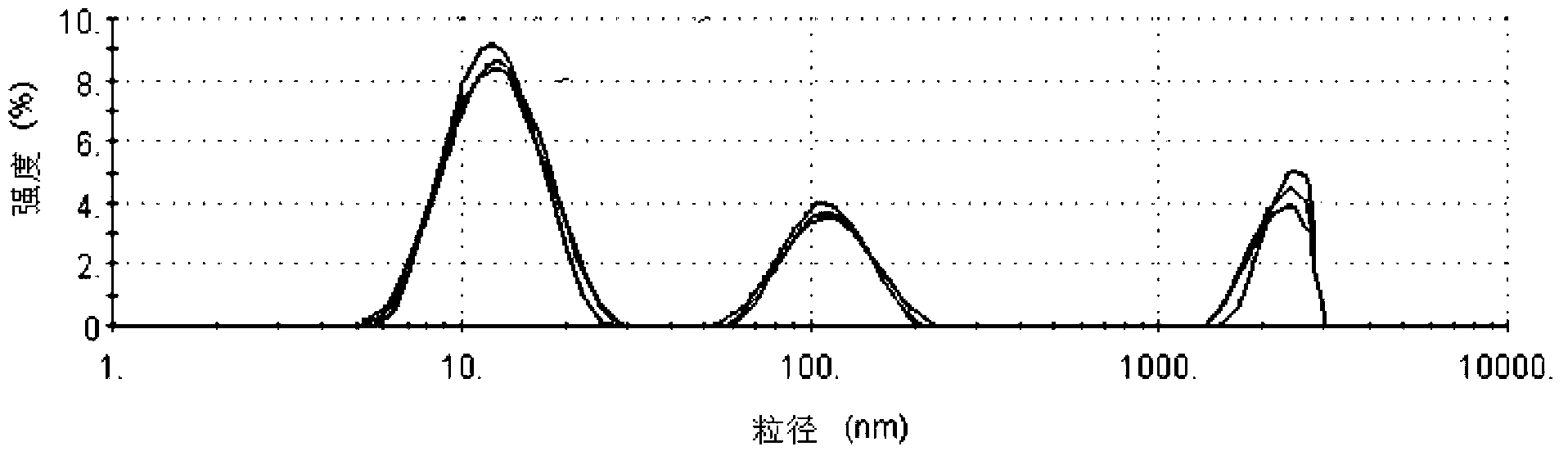
The invention provides a flurbiprofen axetil emulsion for injection and a preparation method thereof. The preparation method comprises the following steps: under the protection of nitrogen, the oil phase is added into the aqueous phase step by step and shearing and mixing for many times to obtain colostrum; 40-60wt% of total oil phase is added into that water phase, shear and mixing are carried out for 10-30min to obtain crude emulsion A, wherein the crude emulsion A is obtained by adding 40-60 wt% of total oil phase into the water phase and mixing for 10-30min; 20-30wt% of total oil phase isadded into that crude emulsion A, and the crude emulsion B is obtained by shear and mixing for 10-30min; Shearing and mixing the crude emulsion B and the remaining oil phase for 10 to 30 minutes; Theoil phase includes flurbiprofen esters, oil phase solvents, emulsifiers and stabilizers, and the aqueous phase includes water for injection and / or osmotic pressure regulators. Under the precondition of the presence of the stabilizer in the oil phase, the invention adopts the oil phase step-by-step and multiple emulsification technology, which can effectively improve the emulsification effect, makethe particle size of the obtained emulsion more uniform, the encapsulation efficiency of the drug higher, and the targeting of the drug to the wound tissue better.
Owner:WUHAN DOCAN PHARMA
Flurbiprofen axetil structure fat emulsion injection liquid and preparation method thereof
InactiveCN108078928ASignificant anti-inflammatory and analgesic effectsQuick effectOrganic active ingredientsAntipyreticCrystallographyTriglyceride
The invention relates to the technical field of a medicine preparation, in particular to flurbiprofen axetil structure fat emulsion injection liquid and a preparation method thereof. The flurbiprofenaxetil structure fat emulsion injection liquid is prepared from flurbiprofen axetil, structure triglyceride, emulsifiers, lecithin, disodium hydrogen phosphate, glycerol, citric acid and injection water. The flurbiprofen axetil structure fat emulsion injection liquid provided by the invention has the advantages that the medicine effect is obvious; the toxic and side effects are lower; the stability is high.
Owner:GUANGDONG JIABO PHARM CO LTD
Method for preparing S-(+)-flurbiprofen axetil high in optical purity
ActiveCN105777544AGuaranteed responseRacemization not foundOrganic active ingredientsAntipyreticOrganic baseReaction temperature
The invention discloses a method for preparing S-(+)-flurbiprofen axetil high in optical purity.The method comprises the steps of 1, making S-(+)-flurbiprofen axetil react with 1-substituted ethyl acetate in the presence of alkali and organic solvent for 3-15 h at the temperature of 0-25 DEG C; 2, extracting and washing a reaction product, and separating out grease; 3, conducting column chromatographic purification on the grease; 4, removing organic solution residues with the solvent coevaporation method, and then conducting vacuum drying to obtain the target product.Inorganic base is not used, organic base highly intersoluble with organic solvent is adopted, a proper solvent ratio is selected to guarantee the proceeding of reaction, and racemization and product breakdown which occur often are avoided during preparation.The total yield of the prepared S-(+)-flurbiprofen axetil can be 80% or more, and optical purity is higher than 99%.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Flurbiprofen axetil microsphere preparation
The invention provides a flurbiprofen axetil microsphere preparation, a preparation method and an application thereof. The plurbiprofen axetil microsphere preparation comprises flurbiprofen axetil, an oil phase solvent and chitosan and / or derivatives thereof and an emulsifying agent. The method for preparing the flurbiprofen axetil microsphere preparation comprises the following steps of: (1) mixing oil phase mixture containing the flurbiprofen axetil, the oil phase solvent and the emulsifying agent so as to produce a uniform oil phase; (2) mixing aqueous phase mixture containing chitosan and / or the derivatives thereof and water so as to form a uniform aqueous phase; (3) adding the oil phase into the aqueous phase so as to form initial emulsion; and (4) homogenizing the initial emulsion. The invention further provides an application of the flurbiprofen axetil microsphere preparation to be used for preparing analgesic or anti-inflammatory drugs.
Owner:WUHAN DOCAN PHARMA
Preparation method of flurbiprofen axetil
ActiveCN103012144BConvenient sourceMild reaction conditionsOrganic compound preparationCarboxylic acid esters preparationPropanoic acidDistillation
Owner:哈药集团股份有限公司 +1
Flurbiprofen axetil microsphere injection and preparation method thereof
InactiveCN104784115AImprove stabilityExtend cycle timeOrganic active ingredientsAntipyreticMicrosphereSodium oleate
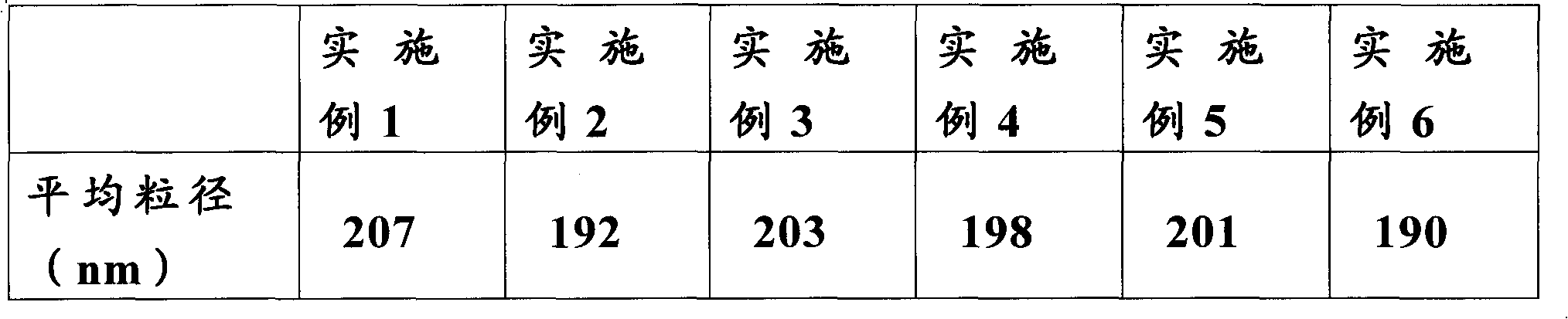
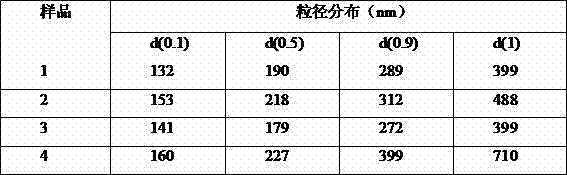
The invention relates to a flurbiprofen axetil microsphere injection and a preparation method thereof. The flurbiprofen axetil microsphere injection is composed of a main drug, long chain oil for injection, one or more emulsifier, a stabilizer, an isotonic agent, a pH adjusting agent and water for injection. The one or more emulsifier is / are safe nontoxic and has good biocompatibility, such as lecithin and F68, and sodium oleate is added as the stabilizer to guarantee the stability of physical and chemical properties before and after disinfection. The particle size of the flurbiprofen axetil microsphere injection is stable and uniform, and is 180-200nm concretely, and the flurbiprofen axetil microsphere injection is mainly used for cancer pain easing and postoperative pain relieving, and is suitable for being widely clinically applied.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Flurbiprofen axetil injection and preparation method thereof
ActiveCN104173279AGood solubilization effectImprove stabilityOrganic active ingredientsAntipyreticSolubilityNephropathy
The invention relates to flurbiprofen axetil injection and a preparation method thereof and belongs to the technical field of pharmaceutic preparations. The flurbiprofen axetil injection contains flurbiprofen axetil and a cosolvent in a mole ratio of 1:(1-1.8), has the advantages of good solubility, stable quality and the like, can be used for avoiding drug-induced risks, probably caused by other products, of patients suffering from heart disease, hypertension and kidney disease and avoiding adverse effects such as nausea and stomachache.
Owner:HEBEI YIPIN PHARMA
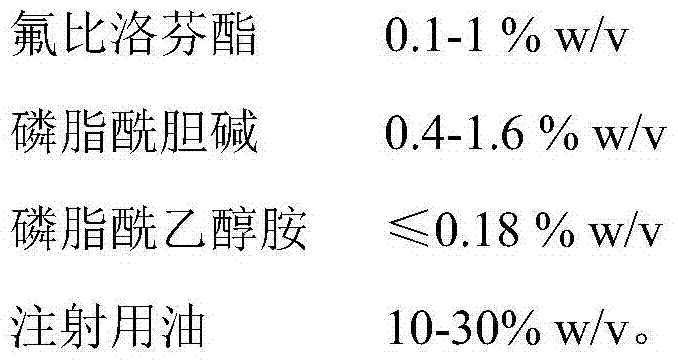
A kind of stable flurbiprofen axetil pharmaceutical composition
ActiveCN104922065BReduce degradationImprove stabilityOrganic active ingredientsAntipyreticMedicineImpurity
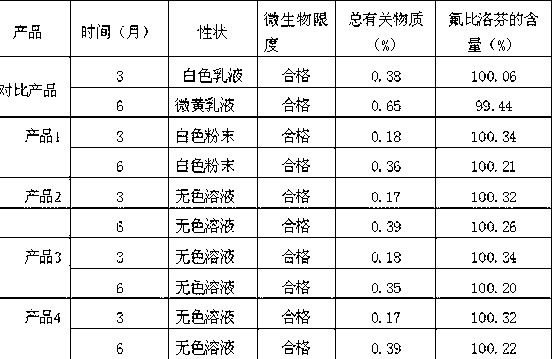
The invention provides a stable flurbiprofen axetil medicine composition which comprises flurbiprofen axetil with the weight / volume percent of 0.1-1% w / v, phosphatidyl choline with the weight / volume percent of 0.4-1.6% w / v, phosphatidyl ethanolamine with the weight / volume percent of smaller than or equal to 0.18% w / v, injection oil with the weight / volume percent of 10-30% w / v and injection water. The composition has good stability, the content of hydrolyzed impurity flurbiprofen is relatively low, and the shelf life of the composition is prolonged from 18 months to 24 months as compared with that of products sold in the market.
Owner:WISDOM PHARM CO LTD
Method for detecting related substances in flurbiprofen axetil drug
The invention discloses a method for detecting related substances in a flurbiprofen axetil drug. A high performance liquid chromatography is adopted, and the mobile phase is a mixed solution of acetonitrile and water. According to the detection method provided by the invention, two pairs of enantiomers of flurbiprofen axetil and two pairs of enantiomers of defluorinated flurbiprofen axetil can beseparated synchronously.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Flurbiprofen axetil pharmaceutical composition for relieving fever
The invention relates to an application of a flurbiprofen axetil pharmaceutical composition in preparing antipyretics. The flurbiprofen axetil pharmaceutical composition consists of flurbiprofen axetil, oil, egg yolk lecithin, glycerol, disodium hydrogen phosphate, citric acid and injection water, wherein the concentration of the flurbiprofen axetil is 0.1-10mg / ml, the concentration of the oil is 100mg / ml and the concentration of the egg yolk lecithin is 12mg / ml. The flurbiprofen axetil pharmaceutical composition disclosed by the invention can be used for relieving fever of a rat caused by dried yeast.
Owner:BEIJING LANDAN PHARMA TECH
Stable flurbiprofen axetil medicine composition
ActiveCN104922065AReduce degradationImprove stabilityOrganic active ingredientsAntipyreticMedicineEthanolamine synthesis
The invention provides a stable flurbiprofen axetil medicine composition which comprises flurbiprofen axetil with the weight / volume percent of 0.1-1% w / v, phosphatidyl choline with the weight / volume percent of 0.4-1.6% w / v, phosphatidyl ethanolamine with the weight / volume percent of smaller than or equal to 0.18% w / v, injection oil with the weight / volume percent of 10-30% w / v and injection water. The composition has good stability, the content of hydrolyzed impurity flurbiprofen is relatively low, and the shelf life of the composition is prolonged from 18 months to 24 months as compared with that of products sold in the market.
Owner:WISDOM PHARM CO LTD
A kind of pharmaceutical composition of flurbiprofen axetil
ActiveCN104434901BUniform particle sizeImprove stabilityOrganic active ingredientsAntipyreticFat emulsionMedicine
The invention belongs to the technical field of medicines and in particular relates to a pharmaceutical composition of flurbiprofen axetil. The pharmaceutical composition of the flurbiprofen axetil is a fat emulsion injection and is prepared from the following components in parts by weight: 0.005-0.015g / ml flurbiprofen axetil, 0.1-0.12g / ml soybean oil, 0.01-0.02g / ml lecithin, 0.001-0.002g / ml vitamin E, 0.01-0.03g / ml glycerol and a proper amount of pH regulator. The fat emulsion injection is uniform in particle size, is good in stability, is capable of greatly relieving degradation of the flurbiprofen axetil so as to reduce generation of other impurities and lower safety hidden dangers and is good in dilution stability of compatibility.
Owner:桂林澳林制药有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com