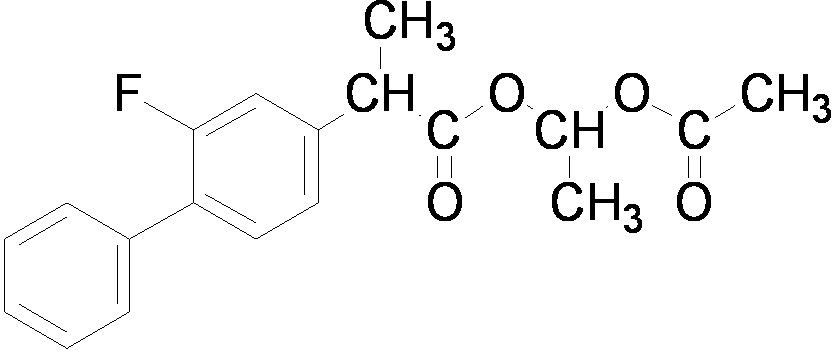
Preparation method of flurbiprofen axetil
The technology of flurbiprofen axetil and flurbiprofen axetil is applied in the preparation field of flurbiprofen axetil, can solve the problems of purification, low heat transfer efficiency and high temperature, and achieves mild reaction conditions, easy-to-obtain raw material sources, and reaction selectivity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
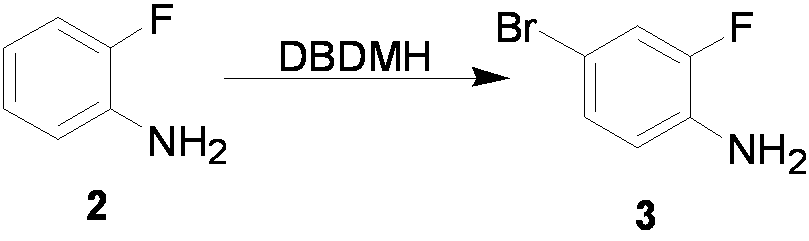
[0042] Example 1 Preparation of compound 4-bromo-2-fluoroaniline
[0043] Dissolve 111.12g (1.0mol) of o-fluoroaniline in 50ml of N,N-dimethylformamide, and lower the reaction temperature to -30°C while stirring. Dissolve 14.30g (0.5mol) DBDMH in 75ml N,N-dimethylformamide, and slowly add it dropwise to the o-fluoroaniline solution, and control the temperature between -25°C and -20°C during the dropwise addition. After the dropwise addition was completed, the reaction was continued for 30 min. Then pour the reaction solution into 500ml of extraction solution (ethyl acetate:n-hexane=1:5), stir and add 300ml of 5% sodium hydroxide aqueous solution, extract and separate layers. The aqueous layer was extracted once more with the extraction solution. The organic layers were combined, washed three times with 100 ml of water, and once with 100 ml of saturated brine. Dry over anhydrous sodium sulfate and concentrate in vacuo to obtain 182 g of the product with a yield of 95.8%.
Embodiment 2
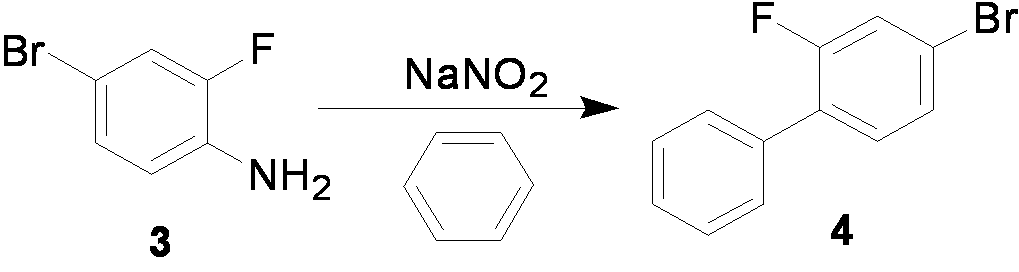
[0044] Example 2 Preparation of 4-bromo-2-fluorobiphenyl
[0045] Add 110.4g (1.6mol) of sodium nitrite into a mixed solution of 250ml of water and 2400ml of benzene, heat to 65°C~70°C, and stir vigorously. Dissolve 152.0g (0.8mol) of compound 3 and 96.1g (1.6mol) of glacial acetic acid in 800ml of benzene, and slowly drop the above mixed solution into the reaction solution, control the speed of dropping, and put the whole dropwise The time is controlled at about 5 hours. After the dropwise addition was completed, the heating reaction was continued for 3 h, and the reaction was stopped. The reaction solution was extracted after cooling down to room temperature, and the organic phase was washed three times with 900 ml of sodium sulfate solution and once with 600 ml of saturated brine. After vacuum concentration, the crude product was dissolved in 800ml 85% sulfuric acid solution, extracted 3 times with 2000ml n-hexane, then the organic phase was washed 5 times with 2000ml 5% ...
Embodiment 3
[0046] Example 3 Preparation of 4-bromo-2-fluorobiphenyl
[0047] Add 110.4g (1.6mol) of sodium nitrite into a mixed solution of 250ml of water and 2400ml of benzene, heat to 65°C~70°C, and stir vigorously. Dissolve 152.0g (0.8mol) of compound 3 and 261.4g (1.6mol) of trichloroacetic acid in 800ml of benzene, and slowly drop the above mixed solution into the reaction solution, control the speed of dropping, and drop the entire The time is controlled at about 5 hours. After the dropwise addition was completed, the heating reaction was continued for 3 h, and the reaction was stopped. The reaction solution was extracted after cooling down to room temperature, and the organic phase was washed three times with 900 ml of sodium sulfate solution and once with 600 ml of saturated brine. After vacuum concentration, the crude product was dissolved in 800ml 85% sulfuric acid solution, extracted 3 times with 2000ml n-hexane, then the organic phase was washed 5 times with 2000ml 5% aqueo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com